Sildenafil triggers tumor lethality through altered expression of HSP90 and degradation of PKD2
- PMID: 31917403
- PMCID: PMC7566345
- DOI: 10.1093/carcin/bgaa001
Sildenafil triggers tumor lethality through altered expression of HSP90 and degradation of PKD2
Abstract
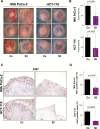
The repurposing of existing drugs has emerged as an attractive additional strategy to the development of novel compounds in the fight against cancerous diseases. Inhibition of phosphodiesterase 5 (PDE5) has been claimed as a potential approach to target various cancer subtypes in recent years. However, data on the treatment of tumors with PDE5 inhibitors as well as the underlying mechanisms are as yet very scarce. Here, we report that treatment of tumor cells with low concentrations of Sildenafil was associated with decreased cancer cell proliferation and augmented apoptosis in vitro and resulted in impaired tumor growth in vivo. Notably, incubation of cancer cells with Sildenafil was associated with altered expression of HSP90 chaperone followed by degradation of protein kinase D2, a client protein previously reported to be involved in tumor growth. Furthermore, the involvement of low doses of PU-H71, an HSP90 inhibitor currently under clinical evaluation, in combination with low concentrations of Sildenafil, synergistically and negatively impacted on the viability of cancer cells in vivo. Taken together, our study suggests that repurposing of already approved drugs, alone or in combination with oncology-dedicated compounds, may represent a novel cancer therapeutic strategy.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





References
-
- Wallis R.M. (1999) The pharmacology of sildenafil, a novel and selective inhibitor of phosphodiesterase (PDE) type 5. Nihon Yakurigaku Zasshi, 114 (suppl. 1), 22P–26P. - PubMed
-
- Bi Y. et al. (2001) The discovery of novel, potent and selective PDE5 inhibitors. Bioorg. Med. Chem. Lett., 11, 2461–2464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

