Understanding Buprenorphine for Use in Chronic Pain: Expert Opinion
- PMID: 31917418
- PMCID: PMC7139205
- DOI: 10.1093/pm/pnz356
Understanding Buprenorphine for Use in Chronic Pain: Expert Opinion
Abstract
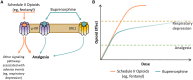
Objective: An expert panel convened to reach a consensus on common misconceptions surrounding buprenorphine, a Schedule III partial µ-opioid receptor agonist indicated for chronic pain. The panel also provided clinical recommendations on the appropriate use of buprenorphine and conversion strategies for switching to buprenorphine from a full µ-opioid receptor agonist for chronic pain management.
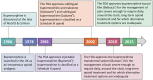
Methods: The consensus panel met on March 25, 2019, to discuss relevant literature and provide recommendations on interpreting buprenorphine as a partial µ-opioid receptor agonist, prescribing buprenorphine before some Schedule II, III, or IV options, perioperative/trauma management of patients taking buprenorphine, and converting patients from a full µ-opioid receptor agonist to buprenorphine.
Results: The panel recommended that buprenorphine's classification as a partial µ-opioid receptor agonist not be clinically translated to mean partial analgesic efficacy. The panel also recommended that buprenorphine be considered before some Schedule II, III, or IV opioids in patients with a favorable risk/benefit profile on the basis of metabolic factors, abuse potential, and tolerability and that buprenorphine be continued during the perioperative/trauma period. In addition, switching patients from a full µ-opioid receptor agonist to buprenorphine should be considered with no weaning period at starting doses that are based on the previous opioid dose.
Conclusions: These recommendations provide a framework for clinicians to address most clinical scenarios regarding buprenorphine use. The overall consensus of the panel was that buprenorphine is a unique Schedule III opioid with favorable pharmacologic properties and a safety profile that may be desirable for chronic pain management.
Keywords: Buprenorphine; Chronic Pain; Opioid; Partial Agonist; Schedule III; µ-Opioid Receptor.
© 2020 American Academy of Pain Medicine.
Figures

References
-
- Dillie KS, Fleming MF, Mundt MP, French MT.. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med 2008;21(2):108–17. - PubMed
-
- Volkow ND, McLellan AT.. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016;374(13):1253–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

