Profiling myelodysplastic syndromes by mass cytometry demonstrates abnormal progenitor cell phenotype and differentiation
- PMID: 31917512
- PMCID: PMC9292828
- DOI: 10.1002/cyto.b.21860
Profiling myelodysplastic syndromes by mass cytometry demonstrates abnormal progenitor cell phenotype and differentiation
Abstract
Background: We sought to enhance the cytometric analysis of myelodysplastic syndromes (MDS) by performing a pilot study of a single cell mass cytometry (MCM) assay to more comprehensively analyze patterns of surface marker expression in patients with MDS.
Methods: Twenty-three MDS and five healthy donor bone marrow samples were studied using a 34-parameter mass cytometry panel utilizing barcoding and internal reference standards. The resulting data were analyzed by both traditional gating and high-dimensional clustering.
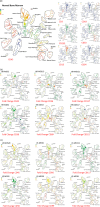
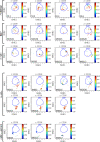
Results: This high-dimensional assay provided three major benefits relative to traditional cytometry approaches: First, MCM enabled detection of aberrant surface maker at high resolution, detecting aberrancies in 27/31 surface markers, encompassing almost every previously reported MDS surface marker aberrancy. Additionally, three previously unrecognized aberrancies in MDS were detected in multiple samples at least one developmental stage: increased CD321 and CD99; and decreased CD47. Second, analysis of the stem and progenitor cell compartment (HSPCs), demonstrated aberrant expression in 21 of the 23 MDS samples, which were not detected in three samples from patients with idiopathic cytopenia of undetermined significance. These immunophenotypically abnormal HSPCs were also the single most significant distinguishing feature between clinical risk groups. Third, unsupervised clustering of high-parameter MCM data allowed identification of abnormal differentiation patterns associated with immunophenotypically aberrant myeloid cells similar to myeloid derived suppressor cells.
Conclusions: These results demonstrate that high-parameter cytometry methods that enable simultaneous analysis of all bone marrow cell types could enhance the diagnostic utility of immunophenotypic analysis in MDS.
© 2020 The Authors. Cytometry Part B: Clinical Cytometry published by Wiley Periodicals, Inc. on behalf of International Clinical Cytometry Society.
Conflict of interest statement
G.K.B. and G.P.N. have provided paid consulting to Fluidigm Sciences. G.P.N. has equity ownership of Fluidigm Sciences. Other authors disclose no potential conflicts of interest.
Figures




References
-
- Bandura, D. R. , Baranov, V. I. , Ornatsky, O. I. , Antonov, A. , Kinach, R. , Lou, X. , … Tanner, S. D. (2009). Mass cytometry: Technique for real time single cell multitarget immunoassay based on inductively coupled plasma time‐of‐flight mass spectrometry. Analytical Chemistry, 81(16), 6813–6822. - PubMed
-
- Behbehani, G. K. , Samusik, N. , Bjornson, Z. B. , Fantl, W. J. , Medeiros, B. C. , & Nolan, G. P. (2015). Mass Cytometric functional profiling of acute myeloid leukemia defines cell‐cycle and Immunophenotypic properties that correlate with known responses to therapy. Cancer Discovery, 5(9), 988–1003. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

