Multimodality Imaging of Dementia: Clinical Importance and Role of Integrated Anatomic and Molecular Imaging
- PMID: 31917652
- PMCID: PMC6996605
- DOI: 10.1148/rg.2020190070
Multimodality Imaging of Dementia: Clinical Importance and Role of Integrated Anatomic and Molecular Imaging
Abstract
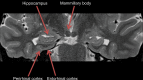
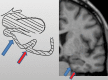
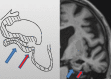
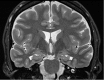
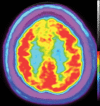
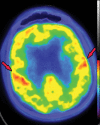
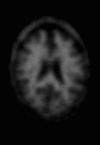
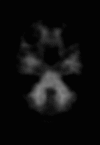
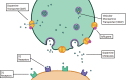
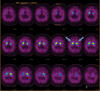
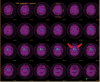
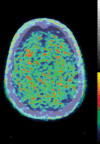
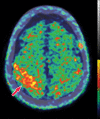
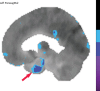
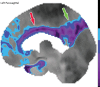
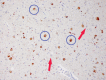
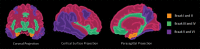
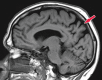
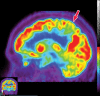
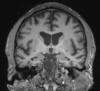
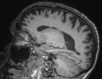
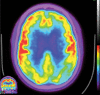
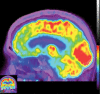
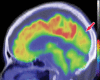
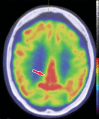
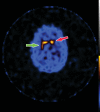
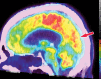
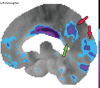
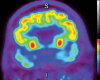
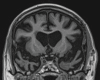
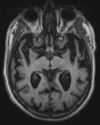
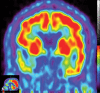
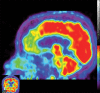
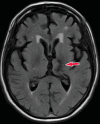
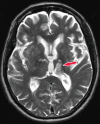
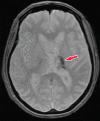
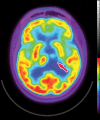
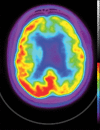
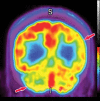
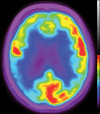
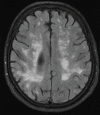
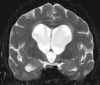
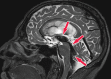
Neurodegenerative diseases are a devastating group of disorders that can be difficult to accurately diagnose. Although these disorders are difficult to manage owing to relatively limited treatment options, an early and correct diagnosis can help with managing symptoms and coping with the later stages of these disease processes. Both anatomic structural imaging and physiologic molecular imaging have evolved to a state in which these neurodegenerative processes can be identified relatively early with high accuracy. To determine the underlying disease, the radiologist should understand the different distributions and pathophysiologic processes involved. High-spatial-resolution MRI allows detection of subtle morphologic changes, as well as potential complications and alternate diagnoses, while molecular imaging allows visualization of altered function or abnormal increased or decreased concentration of disease-specific markers. These methodologies are complementary. Appropriate workup and interpretation of diagnostic studies require an integrated, multimodality, multidisciplinary approach. This article reviews the protocols and findings at MRI and nuclear medicine imaging, including with the use of flurodeoxyglucose, amyloid tracers, and dopaminergic transporter imaging (ioflupane). The pathophysiology of some of the major neurodegenerative processes and their clinical presentations are also reviewed; this information is critical to understand how these imaging modalities work, and it aids in the integration of clinical data to help synthesize a final diagnosis. Radiologists and nuclear medicine physicians aiming to include the evaluation of neurodegenerative diseases in their practice should be aware of and familiar with the multiple imaging modalities available and how using these modalities is essential in the multidisciplinary management of patients with neurodegenerative diseases.©RSNA, 2020.
Figures



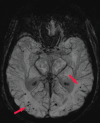
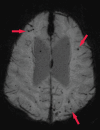
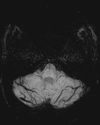
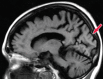
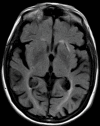
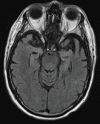

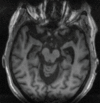
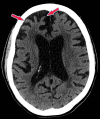
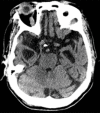
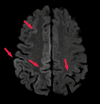
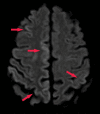
References
-
- Alzheimer’s Association. 2019 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2019;15(3):321-87.
-
- Morris JC, Csernansky J, Price JL. MRI measures of entorhinal cortex versus hippocampus in preclinical AD. Neurology 2002;59(9):1474–1475; author reply 1474–1475. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

