Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota
- PMID: 31917685
- PMCID: PMC7108906
- DOI: 10.1172/JCI133473
Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota
Abstract
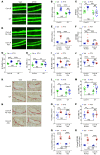
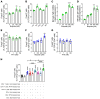
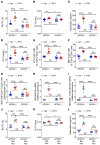
Parathyroid hormone (PTH) is a critical regulator of skeletal development that promotes both bone formation and bone resorption. Using microbiota depletion by wide-spectrum antibiotics and germ-free (GF) female mice, we showed that the microbiota was required for PTH to stimulate bone formation and increase bone mass. Microbiota depletion lowered butyrate levels, a metabolite responsible for gut-bone communication, while reestablishment of physiologic levels of butyrate restored PTH-induced anabolism. The permissive activity of butyrate was mediated by GPR43 signaling in dendritic cells and by GPR43-independent signaling in T cells. Butyrate was required for PTH to increase the number of bone marrow (BM) regulatory T cells (Tregs). Tregs stimulated production of the osteogenic Wnt ligand Wnt10b by BM CD8+ T cells, which activated Wnt-dependent bone formation. Together, these data highlight the role that butyrate produced by gut luminal microbiota plays in triggering regulatory pathways, which are critical for the anabolic action of PTH in bone.
Keywords: Bone Biology; Bone disease; Dendritic cells; T cells.
Conflict of interest statement
Figures




Comment in
-
The microbiome adds to the complexity of parathyroid hormone action on bone.J Clin Invest. 2020 Apr 1;130(4):1615-1617. doi: 10.1172/JCI135712. J Clin Invest. 2020. PMID: 32125288 Free PMC article.
-
Gut microbiota orchestrates PTH action in bone: role of butyrate and T cells.Kidney Int. 2020 Aug;98(2):269-272. doi: 10.1016/j.kint.2020.03.004. Epub 2020 Mar 25. Kidney Int. 2020. PMID: 32600825 No abstract available.
References
-
- Potts J. Chapter 13. In: Avioli LV, Krane S, eds. Metabolic Bone Diseases. San Diego, CA: Academic Press; 1998:411–442.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

