Inhibiting the coregulator CoREST impairs Foxp3+ Treg function and promotes antitumor immunity
- PMID: 31917688
- PMCID: PMC7108912
- DOI: 10.1172/JCI131375
Inhibiting the coregulator CoREST impairs Foxp3+ Treg function and promotes antitumor immunity
Abstract
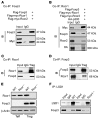
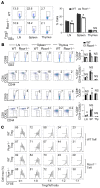
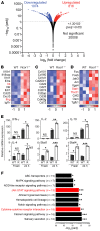
Foxp3+ Tregs are key to immune homeostasis, but the contributions of various large, multiprotein complexes that regulate gene expression remain unexplored. We analyzed the role in Tregs of the evolutionarily conserved CoREST complex, consisting of a scaffolding protein, Rcor1 or Rcor2, plus Hdac1 or Hdac2 and Lsd1 enzymes. Rcor1, Rcor2, and Lsd1 were physically associated with Foxp3, and mice with conditional deletion of Rcor1 in Foxp3+ Tregs had decreased proportions of Tregs in peripheral lymphoid tissues and increased Treg expression of IL-2 and IFN-γ compared with what was found in WT cells. Mice with conditional deletion of the gene encoding Rcor1 in their Tregs had reduced suppression of homeostatic proliferation, inability to maintain long-term allograft survival despite costimulation blockade, and enhanced antitumor immunity in syngeneic models. Comparable findings were seen in WT mice treated with CoREST complex bivalent inhibitors, which also altered the phenotype of human Tregs and impaired their suppressive function. Our data point to the potential for therapeutic modulation of Treg functions by pharmacologic targeting of enzymatic components of the CoREST complex and contribute to an understanding of the biochemical and molecular mechanisms by which Foxp3 represses large gene sets and maintains the unique properties of this key immune cell.
Keywords: Cancer immunotherapy; Cellular immune response; Immunology; Oncology; T cells.
Conflict of interest statement
Figures







Comment in
-
CoRESTed development of regulatory T cells.J Clin Invest. 2020 Apr 1;130(4):1618-1621. doi: 10.1172/JCI135713. J Clin Invest. 2020. PMID: 32125289 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

