Clinical efficacy of irinotecan plus raltitrexed chemotherapy in refractory esophageal squamous cell cancer
- PMID: 31917701
- PMCID: PMC7077961
- DOI: 10.1097/CAD.0000000000000891
Clinical efficacy of irinotecan plus raltitrexed chemotherapy in refractory esophageal squamous cell cancer
Abstract
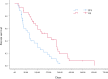
Our retrospective study assessed the efficacy and safety of irinotecan plus raltitrexed in esophageal squamous cell cancer (ESCC) patients who were previously treated with multiple systemic therapies. Between January 2016 and December 2018, records of 38 ESCC patients who underwent irinotecan plus raltitrexed chemotherapy after at least one line of chemotherapy were reviewed. Efficacy assessment was performed every two cycles according to the RECIST version 1.1. A total of 95 cycles of chemotherapy were administered, and the median course was 3 (range 2-6). There was no treatment-related death. Nine patients had partial response, 21 had stable disease and eight had progressive disease. The overall objective response rate was 23.68% (9/38) and the disease control rate was78.94% (30/38). After a median follow-up of 18.5 months, the median progression-free survival and overall survival were 105 and 221 days, respectively. There were five patients (13.15%) with grade 3/4 leukopenia, three patients (7.89%) with grade 3/4 neutropenia and one patient (2.63%) with grade 3/4 diarrhea. The combination of irinotecan plus raltitrexed was effective for pretreated ESCC patients. Further studies are needed to determine the optimal dose of the two drugs.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424 - PubMed
-
- Wang QL, Xie SH, Li WT, Lagergren J. Smoking cessation and risk of esophageal cancer by histological type: systematic review and meta-analysis. J Natl Cancer Inst. 2017; 109 - PubMed
-
- Overman MJ, Kazmi SM, Jhamb J, Lin E, Yao JC, Abbruzzese JL, et al. Weekly docetaxel, cisplatin, and 5-fluorouracil as initial therapy for patients with advanced gastric and esophageal cancer. Cancer. 2010; 116:1446–1453 - PubMed
-
- Takahashi H, Arimura Y, Yamashita K, Okahara S, Tanuma T, Kodaira J, et al. Phase I/II study of docetaxel/cisplatin/fluorouracil combination chemotherapy against metastatic esophageal squamous cell carcinoma. J Thorac Oncol. 2010; 5:122–128 - PubMed

