Transgenic interleukin 11 expression causes cross-tissue fibro-inflammation and an inflammatory bowel phenotype in mice
- PMID: 31917819
- PMCID: PMC6952089
- DOI: 10.1371/journal.pone.0227505
Transgenic interleukin 11 expression causes cross-tissue fibro-inflammation and an inflammatory bowel phenotype in mice
Abstract
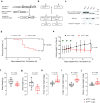
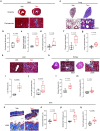
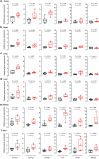
Interleukin 11 (IL11) is a profibrotic cytokine, secreted by myofibroblasts and damaged epithelial cells. Smooth muscle cells (SMCs) also secrete IL11 under pathological conditions and express the IL11 receptor. Here we examined the effects of SMC-specific, conditional expression of murine IL11 in a transgenic mouse (Il11SMC). Within days of transgene activation, Il11SMC mice developed loose stools and progressive bleeding and rectal prolapse, which was associated with a 65% mortality by two weeks. The bowel of Il11SMC mice was inflamed, fibrotic and had a thickened wall, which was accompanied by activation of ERK and STAT3. In other organs, including the heart, lung, liver, kidney and skin there was a phenotypic spectrum of fibro-inflammation, together with consistent ERK activation. To investigate further the importance of stromal-derived IL11 in the inflammatory bowel phenotype we used a second model with fibroblast-specific expression of IL11, the Il11Fib mouse. This additional model largely phenocopied the Il11SMC bowel phenotype. These data show that IL11 secretion from the stromal niche is sufficient to drive inflammatory bowel disease in mice. Given that IL11 expression in colonic stromal cells predicts anti-TNF therapy failure in patients with ulcerative colitis or Crohn's disease, we suggest IL11 as a therapeutic target for inflammatory bowel disease.
Conflict of interest statement
S.A.C. and S.S. are co-inventors of the patent applications ‘Treatment of fibrosis’ (WO/2017/103108). S.A.C., S.S., W.W.L. and B.N. are co-inventors of the patent application ‘Treatment of SMC mediated disease’ (WO/2019/073057). S.A.C. and S.S. are co-founders and shareholders of Enleofen Bio PTE LTD, a company (which S.A.C. is a director of) that develops anti-IL11 therapeutics. All other authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

