Once-Weekly Somapacitan vs Daily GH in Children With GH Deficiency: Results From a Randomized Phase 2 Trial
- PMID: 31917835
- PMCID: PMC7069655
- DOI: 10.1210/clinem/dgz310
Once-Weekly Somapacitan vs Daily GH in Children With GH Deficiency: Results From a Randomized Phase 2 Trial
Erratum in
-
Corrigendum to: "Once-Weekly Somapacitan vs Daily GH in Children With GH Deficiency: Results From a Randomized Phase 2 Trial".J Clin Endocrinol Metab. 2020 Dec 1;105(12):e4982. doi: 10.1210/clinem/dgaa614. J Clin Endocrinol Metab. 2020. PMID: 32905598 Free PMC article. No abstract available.
Abstract
Context: Daily growth hormone (GH) injections can be burdensome for patients and carers. Somapacitan is a long-acting, reversible albumin-binding GH derivative in development for once-weekly administration in patients with growth hormone deficiency (GHD).
Objective: The objective of this study is to evaluate the efficacy, safety, and tolerability of once-weekly somapacitan vs once-daily GH.
Design: REAL 3 is a multicenter, randomized, controlled, double-blind (somapacitan doses), phase 2 study with a 26-week main and 26-week extension phase (NCT02616562).
Setting: This study took place at 29 sites in 11 countries.
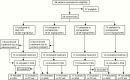
Patients: Fifty-nine GH treatment-naive prepubertal children with GHD were randomly assigned; 58 completed the trial.
Interventions: Interventions comprised 3 somapacitan doses (0.04 [n = 16], 0.08 [n = 15], or 0.16 mg/kg/wk [n = 14]) and daily GH (0.034 mg/kg/d [n = 14]), administered subcutaneously.
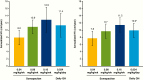
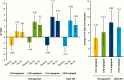
Main outcome measures: The primary end point was height velocity (HV) at week 26. Secondary efficacy end points included HV SD score (SDS) and insulin-like growth factor-I (IGF-I) SDS.
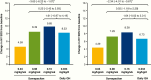
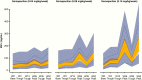
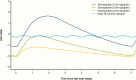
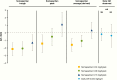
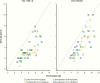
Results: At week 26, mean (SD) annualized HV for the somapacitan groups was 8.0 (2.0), 10.9 (1.9), and 12.9 (3.5) cm/year, respectively, vs 11.4 (3.3) cm/year for daily GH; estimated treatment difference (somapacitan 0.16 mg/kg/week-daily GH): 1.7 [95% CI -0.2 to 3.6] cm/year. HV was sustained at week 52, and significantly greater with somapacitan 0.16 mg/kg/week vs daily GH. Mean (SD) change from baseline in HV SDS at week 52 was 4.72 (2.79), 6.14 (3.36), and 8.60 (3.15) for the somapacitan groups, respectively, vs 7.41 (4.08) for daily GH. Model-derived mean (SD) IGF-I SDS for the somapacitan groups was -1.62 (0.86), -1.09 (0.78), and 0.31 (1.06), respectively, vs -0.40 (1.50) observed for daily GH. Safety and tolerability were consistent with the profile of daily GH.
Conclusions: In children with GHD, once-weekly somapacitan 0.16 mg/kg/week provided the closest efficacy match with similar safety and tolerability to daily GH after 26 and 52 weeks of treatment. A short visual summary of our work is available (1).
Keywords: growth hormone; growth hormone deficiency; growth hormone replacement therapy; long-acting growth hormone; somapacitan; treatment burden.
© Endocrine Society 2020.
Figures



Comment in
-
Long-acting Growth Hormone Therapy: A REAL3 Alternative to Daily Growth Hormone Treatment?J Clin Endocrinol Metab. 2020 Apr 1;105(4):dgaa074. doi: 10.1210/clinem/dgaa074. J Clin Endocrinol Metab. 2020. PMID: 32055832 No abstract available.
References
-
- https://nn-product.videomarketingplatform.co/secret/61057287/48b6ad3a853... https://nn-product.videomarketingplatform.co/secret/61057287/48b6ad3a853... Sävendahl L, Battelino T, Brod M, et al. Animated summary.
-
- Grimberg A, DiVall SA, Polychronakos C, et al. ; Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86(6):361–397. - PubMed
-
- Yuen KCJ, Miller BS, Biller BMK. The current state of long-acting growth hormone preparations for growth hormone therapy. Curr Opin Endocrinol Diabetes Obes. 2018;25(4):267–273. - PubMed
-
- Kapoor RR, Burke SA, Sparrow SE, et al. . Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93(2):147–148. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

