Dynamics of human monocytes and airway macrophages during healthy aging and after transplant
- PMID: 31917836
- PMCID: PMC7062517
- DOI: 10.1084/jem.20191236
Dynamics of human monocytes and airway macrophages during healthy aging and after transplant
Abstract
The ontogeny of airway macrophages (AMs) in human lung and their contribution to disease are poorly mapped out. In mice, aging is associated with an increasing proportion of peripherally, as opposed to perinatally derived AMs. We sought to understand AM ontogeny in human lung during healthy aging and after transplant. We characterized monocyte/macrophage populations from the peripheral blood and airways of healthy volunteers across infancy/childhood (2-12 yr), maturity (20-50 yr), and older adulthood (>50 yr). Single-cell RNA sequencing (scRNA-seq) was performed on airway inflammatory cells isolated from sex-mismatched lung transplant recipients. During healthy aging, the proportions of blood bronchoalveolar lavage (BAL) classical monocytes peak in adulthood and decline in older adults. scRNA-seq of BAL cells from lung transplant recipients indicates that after transplant, the majority of AMs are recipient derived. These data show that during aging, the peripheral monocyte phenotype is consistent with that found in the airways and, furthermore, that the majority of human AMs after transplant are derived from circulating monocytes.
© 2020 Byrne et al.
Conflict of interest statement
Disclosures: Dr. Molyneaux reported grants from Boehringer Ingelheim, personal fees from Hoffman-La Roche, and grants from AstraZeneca outside the submitted work. Dr. Chambers reported "other" from Seqbio Pty Ltd outside the submitted work. No other disclosures were reported.
Figures

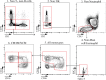
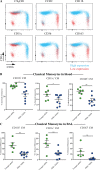
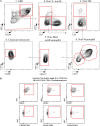
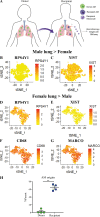
References
-
- Allden S.J., Ogger P.P., Ghai P., McErlean P., Hewitt R., Toshner R., Walker S.A., Saunders P., Kingston S., Molyneaux P.L., et al. . 2019. The Transferrin Receptor CD71 Delineates Functionally Distinct Airway Macrophage Subsets during Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 200:209–219. 10.1164/rccm.201809-1775OC - DOI - PMC - PubMed
-
- Bossley C.J., Fleming L., Gupta A., Regamey N., Frith J., Oates T., Tsartsali L., Lloyd C.M., Bush A., and Saglani S.. 2012. Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J. Allergy Clin. Immunol. 129:974–82.e13. 10.1016/j.jaci.2012.01.059 - DOI - PMC - PubMed

