Properties of Aqueous Trehalose Mixtures: Glass Transition and Hydrogen Bonding
- PMID: 31917927
- PMCID: PMC7467637
- DOI: 10.1021/acs.jctc.9b01071
Properties of Aqueous Trehalose Mixtures: Glass Transition and Hydrogen Bonding
Abstract
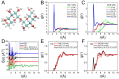
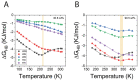
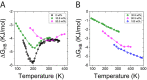
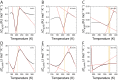
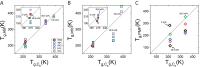
Trehalose is a naturally occurring disaccharide known to remarkably stabilize biomacromolecules in the biologically active state. The stabilizing effect is typically observed over a large concentration range and affects many macromolecules including proteins, lipids, and DNA. Of special interest is the transition from aqueous solution to the dense and highly concentrated glassy state of trehalose that has been implicated in bioadaptation of different organisms toward desiccation stress. Although several mechanisms have been suggested to link the structure of the low water content glass with its action as an exceptional stabilizer, studies are ongoing to resolve which are most pertinent. Specifically, the role that hydrogen bonding plays in the formation of the glass is not well resolved. Here we model aqueous trehalose mixtures over a wide concentration range, using molecular dynamics simulations with two available force fields. Both force fields indicate glass transition temperatures and osmotic pressures that are close to experimental values, particularly at high trehalose contents. We develop and employ a methodology that allows us to analyze the thermodynamics of hydrogen bonds in simulations at different water contents and temperatures. Remarkably, this analysis is able to link the liquid to glass transition with changes in hydrogen bond characteristics. Most notably, the onset of the glassy state can be quantitatively related to the transition from weakly to strongly correlated hydrogen bonds. Our findings should help resolve the properties of the glass and the mechanisms of its formation in the presence of added macromolecules.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
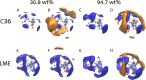


References
-
- Hochachka P. W.; Somero G. N.. Biochemical Adaptation: Mechanisms and Process in Physiology Evolution; Oxford: New York, 2002; pp 217–289.
LinkOut - more resources
Full Text Sources
