Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice
- PMID: 31918577
- PMCID: PMC7093230
- DOI: 10.1161/CIRCULATIONAHA.119.042640
Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice
Abstract
Background: Diabetes mellitus exacerbates myocardial ischemia/reperfusion (MI/R) injury by incompletely understood mechanisms. Adipocyte dysfunction contributes to remote organ injury. However, the molecular mechanisms linking dysfunctional adipocytes to increased MI/R injury remain unidentified. The current study attempted to clarify whether and how small extracellular vesicles (sEV) may mediate pathological communication between diabetic adipocytes and cardiomyocytes, exacerbating MI/R injury.
Methods: Adult male mice were fed a normal or a high-fat diet for 12 weeks. sEV (from diabetic serum, diabetic adipocytes, or high glucose/high lipid-challenged nondiabetic adipocytes) were injected intramyocardially distal of coronary ligation. Animals were subjected to MI/R 48 hours after injection.
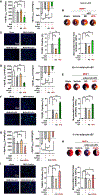
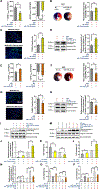
Results: Intramyocardial injection of diabetic serum sEV in the nondiabetic heart significantly exacerbated MI/R injury, as evidenced by poorer cardiac function recovery, larger infarct size, and greater cardiomyocyte apoptosis. Similarly, intramyocardial or systemic administration of diabetic adipocyte sEV or high glucose/high lipid-challenged nondiabetic adipocyte sEV significantly exacerbated MI/R injury. Diabetic epididymal fat transplantation significantly increased MI/R injury in nondiabetic mice, whereas administration of a sEV biogenesis inhibitor significantly mitigated MI/R injury in diabetic mice. A mechanistic investigation identified that miR-130b-3p is a common molecule significantly increased in diabetic serum sEV, diabetic adipocyte sEV, and high glucose/high lipid-challenged nondiabetic adipocyte sEV. Mature (but not primary) miR-130b-3p was significantly increased in the diabetic and nondiabetic heart subjected to diabetic sEV injection. Whereas intramyocardial injection of a miR-130b-3p mimic significantly exacerbated MI/R injury in nondiabetic mice, miR-130b-3p inhibitors significantly attenuated MI/R injury in diabetic mice. Molecular studies identified AMPKα1/α2, Birc6, and Ucp3 as direct downstream targets of miR-130b-3p. Overexpression of these molecules (particularly AMPKα2) reversed miR-130b-3p induced proapoptotic/cardiac harmful effect. Finally, miR-130b-3p levels were significantly increased in plasma sEV from patients with type 2 diabetes mellitus. Incubation of cardiomyocytes with diabetic patient sEV significantly exacerbated ischemic injury, an effect blocked by miR-130b-3p inhibitor.
Conclusions: We demonstrate for the first time that miR-130b-3p enrichment in dysfunctional adipocyte-derived sEV, and its suppression of multiple antiapoptotic/cardioprotective molecules in cardiomyocytes, is a novel mechanism exacerbating MI/R injury in the diabetic heart. Targeting miR-130b-3p mediated pathological communication between dysfunctional adipocytes and cardiomyocytes may be a novel strategy attenuating diabetic exacerbation of MI/R injury.
Keywords: apoptosis; diabetes mellitus; extracellular vesicles; ischemia-reperfusion injury; microRNA.
Figures






Comment in
-
Letter by Li et al Regarding Article, "Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice".Circulation. 2020 Aug 18;142(7):e95-e96. doi: 10.1161/CIRCULATIONAHA.120.047919. Epub 2020 Aug 17. Circulation. 2020. PMID: 32804561 No abstract available.
-
Letter by Ren et al Regarding Article, "Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice".Circulation. 2020 Aug 18;142(7):e97-e98. doi: 10.1161/CIRCULATIONAHA.120.048393. Epub 2020 Aug 17. Circulation. 2020. PMID: 32804562 No abstract available.
-
Response by Gan et al to Letter Regarding Article, "Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice".Circulation. 2020 Aug 18;142(7):e99-e100. doi: 10.1161/CIRCULATIONAHA.120.048689. Epub 2020 Aug 17. Circulation. 2020. PMID: 32804566 Free PMC article. No abstract available.
References
-
- Oikonomou EK and Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. 2019;16:83–99. - PubMed
-
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG and Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. - PubMed
-
- Katayama T, Nakashima H, Takagi C, Honda Y, Suzuki S, Iwasaki Y and Yano K. Clinical outcomes and left ventricular function in diabetic patients with acute myocardial infarction treated by primary coronary angioplasty. Int Heart J. 2005;46:607–618. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

