Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer
- PMID: 31918721
- PMCID: PMC6950820
- DOI: 10.1186/s12951-019-0563-2
Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer
Abstract
Background: 5-Fluorouracil (5-FU) has been commonly prescribed for patients with colorectal cancer (CRC), but resistance to 5-FU is one of the main reasons for failure in CRC. Recently, microRNAs (miRNAs) have been established as a means of reversing the dilemma by regulating signaling pathways involved in initiation and progression of CRC. However, how to safely and effectively deliver miRNA to target cells becomes a main challenge.
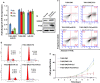
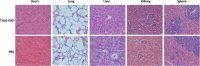
Results: In this study, Engineered exosomes were exploited to simultaneously deliver an anticancer drug 5-FU and miR-21 inhibitor oligonucleotide (miR-21i) to Her2 expressing cancer cells. Purified engineered exosomes from the donor cells loaded with 5-FU and miR-21i via electroporation to introduce into 5-FU-resistant colorectal cancer cell line HCT-1165FR. Furthermore, systematic administration of 5-FU and miR-21i loaded exosomes in tumor bearing mice indicated a significantly anti-tumor effect. The results showed that the engineered exosome-based 5-FU and miR-21i co-delivery system could efficiently facilitate cellular uptake and significantly down-regulate miR-21 expression in 5-FU resistant HCT-1165FR cell lines. Consequently, the down-regulation of miR-21 induced cell cycle arrest, reduced tumor proliferation, increased apoptosis and rescued PTEN and hMSH2 expressions, regulatory targets of miR-21. Of particular importance was the significant reduction in tumor growth in a mouse model of colon cancer with systematic administration of the targeting miR-21i. More excitedly, the combinational delivery of miR-21i and 5-FU with the engineered exosomes effectively reverse drug resistance and significantly enhanced the cytotoxicity in 5-FU-resistant colon cancer cells, compared with the single treatment with either miR-21i or 5-FU.
Conclusion: The strategy for co-delivering the functional small RNA and anticancer drug by exosomes foreshadows a potential approach to reverse the drug resistance in CRC and thus to enhance the efficacy of the cancer treatment.
Keywords: 5-FU; Delivery system; Drug resistance; Exosomes; miR-21 inhibitor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Wang S, Su X, Bai H, Zhao J, Duan J, An T, Zhuo M, Wang Z, Wu M, Li Z, et al. Identification of plasma microRNA profiles for primary resistance to EGFR-TKIs in advanced non-small cell lung cancer (NSCLC) patients with EGFR activating mutation. J Hematol Oncol. 2015;8:127. doi: 10.1186/s13045-015-0210-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

