Innate immune cells, major protagonists of sickle cell disease pathophysiology
- PMID: 31919091
- PMCID: PMC7012475
- DOI: 10.3324/haematol.2019.229989
Innate immune cells, major protagonists of sickle cell disease pathophysiology
Abstract
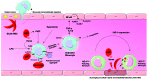
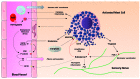
Sickle cell disease (SCD), considered the most common monogenic disease worldwide, is a severe hemoglobin disorder. Although the genetic and molecular bases have long been characterized, the pathophysiology remains incompletely elucidated and therapeutic options are limited. It has been increasingly suggested that innate immune cells, including monocytes, neutrophils, invariant natural killer T cells, platelets and mast cells, have a role in promoting inflammation, adhesion and pain in SCD. Here we provide a thorough review of the involvement of these novel, major protagonists in SCD pathophysiology, highlighting recent evidence for innovative therapeutic perspectives.
Copyright© 2020 Ferrata Storti Foundation.
Figures


References
-
- Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311–323. - PubMed
-
- Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561–1573. - PubMed
-
- Wongtong N, Jones S, Deng Y, Cai J, Ataga KI. Monocytosis is associated with hemolysis in sickle cell disease. Hematology. 2015;20(10):593–597. - PubMed
-
- Sultana C, Shen Y, Rattan V, Johnson C, Kalra VK. Interaction of sickle erythrocytes with endothelial cells in the presence of endothelial cell conditioned medium induces oxidant stress leading to transendothelial migration of monocytes. Blood. 1998;92(10):3924–3935. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

