Predictive Blood-Based Biomarkers in Patients with Epithelial Ovarian Cancer Treated with Carboplatin and Paclitaxel with or without Bevacizumab: Results from GOG-0218
- PMID: 31919136
- PMCID: PMC7073274
- DOI: 10.1158/1078-0432.CCR-19-0226
Predictive Blood-Based Biomarkers in Patients with Epithelial Ovarian Cancer Treated with Carboplatin and Paclitaxel with or without Bevacizumab: Results from GOG-0218
Abstract
Purpose: GOG-0218, a double-blind placebo-controlled phase III trial, compared carboplatin and paclitaxel with placebo, bevacizumab followed by placebo, or bevacizumab followed by bevacizumab in advanced epithelial ovarian cancer (EOC). Results demonstrated significantly improved progression-free survival (PFS), but no overall survival (OS) benefit with bevacizumab. Blood samples were collected for biomarker analyses.
Experimental design: Plasma samples were analyzed via multiplex ELISA technology for seven prespecified biomarkers [IL6, Ang-2, osteopontin (OPN), stromal cell-derived factor-1 (SDF-1), VEGF-D, IL6 receptor (IL6R), and GP130]. The predictive value of each biomarker with respect to PFS and OS was assessed using a protein marker by treatment interaction term within the framework of a Cox proportional hazards model. Prognostic markers were identified using Cox models adjusted for baseline covariates.
Results: Baseline samples were available from 751 patients. According to our prespecified analysis plan, IL6 was predictive of a therapeutic advantage with bevacizumab for PFS (P = 0.007) and OS (P = 0.003). IL6 and OPN were found to be negative prognostic markers for both PFS and OS (P < 0.001). Patients with high median IL6 levels (dichotomized at the median) treated with bevacizumab had longer PFS (14.2 vs. 8.7 months) and OS (39.6 vs. 33.1 months) compared with placebo.
Conclusions: The inflammatory cytokine IL6 may be predictive of therapeutic benefit from bevacizumab when combined with carboplatin and paclitaxel. Aligning with results observed in patients with renal cancer treated with antiangiogenic therapies, it appears plasma IL6 may also define those patients with EOC more or less likely to benefit from the addition of bevacizumab to standard chemotherapy.
©2020 American Association for Cancer Research.
Figures
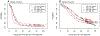

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. - PubMed
-
- Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. - PubMed
-
- Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–45. - PMC - PubMed
-
- Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- U24 CA114793/CA/NCI NIH HHS/United States
- U10 CA027469/CA/NCI NIH HHS/United States
- UG1 CA233191/CA/NCI NIH HHS/United States
- P01 CA142538/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
- R21 CA185730/CA/NCI NIH HHS/United States
- UG1 CA189867/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- UG1 CA233193/CA/NCI NIH HHS/United States
- U10 CA037517/CA/NCI NIH HHS/United States
- U24 CA196067/CA/NCI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

