Hormonal contraception and HIV acquisition among women: an updated systematic review
- PMID: 31919239
- PMCID: PMC6978562
- DOI: 10.1136/bmjsrh-2019-200509
Hormonal contraception and HIV acquisition among women: an updated systematic review
Abstract
Objective: To update a 2016 systematic review on hormonal contraception use and HIV acquisition.
Methods: We searched Pubmed and Embase between 15 January 2016 and 26 June 2019 for longitudinal studies comparing incident HIV infection among women using a hormonal contraceptive method and either non-users or users of another specific hormonal contraceptive method. We extracted information from newly identified studies, assessed study quality, and updated forest plots and meta-analyses.
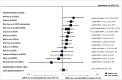
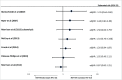
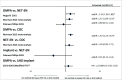
Results: In addition to 31 previously included studies, five more were identified; three provided higher quality evidence. A randomised clinical trial (RCT) found no statistically significant differences in HIV risk among users of intramuscular depot medroxyprogesterone acetate (DMPA-IM), levonorgestrel implant (LNG implant) or the copper intrauterine device (Cu-IUD). An observational study found no statistically significant differences in HIV risk among women using DMPA, norethisterone enanthate (NET-EN), implants (type not specified) or Cu-IUD. Updated results from a previously included observational study continued to find a statistically significant increased HIV risk with oral contraceptives and DMPA compared with no contraceptive use, and found no association between LNG implant and HIV risk.
Conclusions: High-quality RCT data comparing use of DMPA, LNG implant and Cu-IUD does not support previous concerns from observational studies that DMPA-IM use increases the risk of HIV acquisition. Use of other hormonal contraceptive methods (oral contraceptives, NET-EN and implants) is not associated with an increased risk of HIV acquisition.
Keywords: epidemiology; hormonal contraception; human immunodeficiency virus; intrauterine devices.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MIR reports personal fees from Merck (contraceptive implant trainer) and Bayer (IUD trainer), outside the submitted work. TC, JK and PS were members of the ECHO trial consortium, and JK and PS were part of the writing group for the ECHO trial results. All of the authors participated in the 2019 WHO Guideline Development Group (GDG) process which assessed recommendations on contraception for women at high risk of HIV.
Figures

Comment in
-
Contraception and HIV: an exercise in clarity.BMJ Sex Reprod Health. 2020 Jan;46(1):2-3. doi: 10.1136/bmjsrh-2019-200557. BMJ Sex Reprod Health. 2020. PMID: 31919238 No abstract available.
References
-
- World Health Organization (WHO) Hormonal contraceptive eligibility for women at high risk of HIV. Geneva, Switzerland: WHO, 2017.
-
- World Health Organization (WHO) World Health Organization medical eligibility criteria for contraceptive use. 5th edn Geneva, Switzerland: WHO, 2015.
-
- Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet 2019;394:303–13. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
