Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells
- PMID: 31919420
- PMCID: PMC6952363
- DOI: 10.1038/s41598-019-56542-4
Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells
Abstract
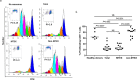
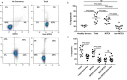
Melanoma patients' plasma contains exosomes produced by malignant and normal cells. Plasma exosomes were isolated and separated by immunocapture into two fractions: melanoma cell-derived exosomes (MTEX) and normal cell-derived exosomes (non-MTEX). Immunosuppressive effects of MTEX on primary human immune cells were evaluated. Exosomes were isolated from plasma of 12 melanoma patients and six healthy donors (HDs). Expression levels of 19 immunoregulatory proteins in MTEX, non-MTEX and HDs exosomes were evaluated by on-bead flow cytometry. Functional/phenotypic changes induced in CD8+ T or natural killer (NK) cells by MTEX or non-MTEX were compared. Plasma protein levels were higher in patients than HDs (P < 0.0009). In patients, MTEX accounted for 23-66% of total exosomes. MTEX were enriched in immunosuppressive proteins (P = 0.03). MTEX, but not HDs exosomes, inhibited CD69 expression (P ≤ 0.0008), induced apoptosis (P ≤ 0.0009) and suppressed proliferation (P ≤ 0.002) in CD8+ T cells and downregulated NKG2D expression in NK cells (P = 0.001). Non-MTEX were enriched in immunostimulatory proteins (P = 0.002) and were only weakly immunosuppressive. Elevated MTEX/total exosome ratios and, surprisingly, non-MTEX ability to induce apoptosis of CD8+ T cells emerged as positive correlates of disease stage. MTEX emerge as the major mechanism of tumor-induced immune suppression and as an underestimated barrier to successful melanoma immunotherapy.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

