Plasmodium asexual growth and sexual development in the haematopoietic niche of the host
- PMID: 31919479
- PMCID: PMC7223625
- DOI: 10.1038/s41579-019-0306-2
Plasmodium asexual growth and sexual development in the haematopoietic niche of the host
Abstract
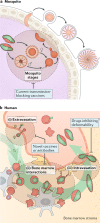
Plasmodium spp. parasites are the causative agents of malaria in humans and animals, and they are exceptionally diverse in their morphology and life cycles. They grow and develop in a wide range of host environments, both within blood-feeding mosquitoes, their definitive hosts, and in vertebrates, which are intermediate hosts. This diversity is testament to their exceptional adaptability and poses a major challenge for developing effective strategies to reduce the disease burden and transmission. Following one asexual amplification cycle in the liver, parasites reach high burdens by rounds of asexual replication within red blood cells. A few of these blood-stage parasites make a developmental switch into the sexual stage (or gametocyte), which is essential for transmission. The bone marrow, in particular the haematopoietic niche (in rodents, also the spleen), is a major site of parasite growth and sexual development. This Review focuses on our current understanding of blood-stage parasite development and vascular and tissue sequestration, which is responsible for disease symptoms and complications, and when involving the bone marrow, provides a niche for asexual replication and gametocyte development. Understanding these processes provides an opportunity for novel therapies and interventions.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- WHO. World Malaria Report 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/repor... (2018).

