Treatment of Perianal Fistulas in Crohn's Disease, Seton Versus Anti-TNF Versus Surgical Closure Following Anti-TNF [PISA]: A Randomised Controlled Trial
- PMID: 31919501
- PMCID: PMC7476637
- DOI: 10.1093/ecco-jcc/jjaa004
Treatment of Perianal Fistulas in Crohn's Disease, Seton Versus Anti-TNF Versus Surgical Closure Following Anti-TNF [PISA]: A Randomised Controlled Trial
Abstract
Background and aims: Most patients with perianal Crohn's fistula receive medical treatment with anti-tumour necrosis factor [TNF], but the results of anti-TNF treatment have not been directly compared with chronic seton drainage or surgical closure. The aim of this study was to assess if chronic seton drainage for patients with perianal Crohn's disease fistulas would result in less re-interventions, compared with anti-TNF and compared with surgical closure.
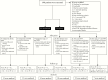
Methods: This randomised trial was performed in 19 European centres. Patients with high perianal Crohn's fistulas with a single internal opening were randomly assigned to: i] chronic seton drainage for 1 year; ii] anti-TNF therapy for 1 year; and iii] surgical closure after 2 months under a short course anti-TNF. The primary outcome was the cumulative number of patients with fistula-related re-intervention[s] at 1.5 years. Patients declining randomisation due to a specific treatment preference were included in a parallel prospective PISA registry cohort.
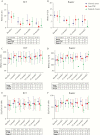
Results: Between September 14, 2013 and November 20, 2017, 44 of the 126 planned patients were randomised. The study was stopped by the data safety monitoring board because of futility. Seton treatment was associated with the highest re-intervention rate [10/15, versus 6/15 anti-TNF and 3/14 surgical closure patients, p = 0.02]. No substantial differences in perianal disease activity and quality of life between the three treatment groups were observed. Interestingly, in the PISA prospective registry, inferiority of chronic seton treatment was not observed for any outcome measure.
Conclusions: The results imply that chronic seton treatment should not be recommended as the sole treatment for perianal Crohn's fistulas.
Keywords: Crohn’s disease; anti-TNF; perianal fistula.
© European Crohn’s and Colitis Organisation (ECCO) 2020.
Figures
Comment in
-
Gleaning insight from the PISA trials.Lancet Gastroenterol Hepatol. 2022 Jul;7(7):587-588. doi: 10.1016/S2468-1253(22)00162-5. Lancet Gastroenterol Hepatol. 2022. PMID: 35709815 No abstract available.
References
-
- Viganò C, Losco A, Caprioli F, Basilisco G. Incidence and clinical outcomes of intersphincteric abscesses diagnosed by anal ultrasonography in patients with Crohn’s disease. Inflamm Bowel Dis 2011;17:2102–8. - PubMed
-
- Hakkaart- van Roijen L, Tan SS. Handleiding voor kostenonderzoek: methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. College voor zorgverzekeringen 2010. zorginstituutnederland.nl.
-
- de Groof EJ, Cabral VN, Buskens CJ, et al. . Systematic review of evidence and consensus on perianal fistula: an analysis of national and international guidelines. Colorectal Dis 2016;18:O119–34. - PubMed
-
- Present DH, Rutgeerts P, Targan S, et al. . Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999;340:1398–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

