Critical Differences Between Dietary Supplement and Prescription Omega-3 Fatty Acids: A Narrative Review
- PMID: 31919792
- PMCID: PMC6999166
- DOI: 10.1007/s12325-019-01211-1
Critical Differences Between Dietary Supplement and Prescription Omega-3 Fatty Acids: A Narrative Review
Abstract
Introduction: Currently available omega-3 (OM-3) fatty acid products in the US are either nonprescription dietary supplements (e.g., fish oils) or prescription (Rx) medications. As such, we aimed to describe critical therapeutic differences among the OM-3 fatty acids, focusing on differences between fish oil supplements and Rx OM-3s.
Methods: A narrative review of known papers salient to this topic was conducted.
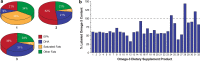
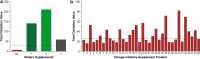
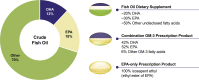
Results: Despite the multiple purported clinical benefits, the published evidence for OM-3 dietary supplements is generally insufficient, inconsistent, or negative. Rx OM-3 products are indicated as an adjunct to diet to reduce triglycerides (TG) in adults with severe hypertriglyceridemia (TG ≥ 500 mg/dl). Recently, the Rx eicosapentaenoic acid (EPA)-only OM-3, icosapent ethyl, demonstrated cardiovascular (CV) risk reduction among statin-treated patients at high risk of CV disease in a large CV outcomes trial (CVOT), and is now also indicated as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated TG (≥ 150 mg/dL) and established CVD or diabetes mellitus and ≥ 2 additional risk factors for CVD. In contrast to the rigorous regulatory standards for safety, efficacy, and manufacturing of medications (whether Rx or over the counter), the Food and Drug Administration manages dietary supplements as food. Issues specific to OM-3 dietary supplements include variable content, labeling inconsistencies, and poor product quality/impurity. Given these issues, OM-3 dietary supplements should not be substituted for Rx OM-3 products. The efficacy of the EPA-only Rx OM-3 product in a large CVOT cannot be extrapolated to other OM-3 products.
Conclusion: Consumers and health care providers need to recognize critical differences between Rx and OM-3 dietary supplements to ensure appropriate use of each OM-3 product.
Keywords: Cardiovascular disease; Dietary supplements; Docosahexaenoic acid; Eicosapentaenoic acid; Fish oils; Hypertriglyceridemia; Icosapent ethyl; Omega-3-fatty acids.
Conflict of interest statement
Daniel E. Hilleman has served on the speakers bureau for Amgen and Amarin and as a consultant for Heron Therapeutics. Barbara S. Wiggins has nothing to disclose. Michael B. Bottorff has served on the speakers bureau for Pfizer and BMS and as a consultant for Medisync.
Figures
Comment in
-
A Response to: Letter to the Editor Regarding "Critical Differences Between Dietary Supplement and Prescription Omega-3 Fatty Acids: a Narrative Review".Adv Ther. 2020 Sep;37(9):4046-4048. doi: 10.1007/s12325-020-01420-z. Epub 2020 Jul 9. Adv Ther. 2020. PMID: 32647913 Free PMC article. No abstract available.
-
Letter to the Editor Regarding Critical Differences Between Dietary Supplement and Prescription Omega-3 Fatty Acids: A Narrative Review.Adv Ther. 2020 Sep;37(9):4043-4045. doi: 10.1007/s12325-020-01421-y. Epub 2020 Jul 9. Adv Ther. 2020. PMID: 32647914 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

