Clinicopathological characteristics of histiocytic sarcoma affecting the central nervous system in dogs
- PMID: 31919895
- PMCID: PMC7096655
- DOI: 10.1111/jvim.15673
Clinicopathological characteristics of histiocytic sarcoma affecting the central nervous system in dogs
Abstract
Background: Histiocytic sarcoma affecting the central nervous system (CNS HS) in dogs may present as primary or disseminated disease, often characterized by inflammation. Prognosis is poor, and imaging differentiation from other CNS tumors can be problematic.
Objective: To characterize the clinicopathological inflammatory features, breed predisposition, and survival in dogs with CNS HS.
Animals: One hundred two dogs with HS, 62 dogs with meningioma.
Methods: Retrospective case series. Records were reviewed for results of cerebrospinal fluid (CSF) analysis, CBC, treatment, and outcome data.
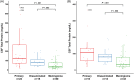
Results: Predisposition for CNS HS was seen in Bernese Mountain Dogs, Golden Retrievers, Rottweilers, Corgis, and Shetland Sheepdogs (P ≤ .001). Corgis and Shetland Sheepdogs had predominantly primary tumors; Rottweilers had exclusively disseminated tumors. Marked CSF inflammation was characteristic of primary rather than disseminated HS, and neoplastic cells were detected in CSF of 52% of affected dogs. Increased neutrophil to lymphocyte ratios were seen in all groups relative to controls (P <.008) but not among tumor subtypes. Definitive versus palliative treatment resulted in improved survival times (P < .001), but overall prognosis was poor.
Conclusions and clinical importance: Clinicopathological differences between primary and disseminated HS suggest that tumor biological behavior and origin may be different. Corgis and Shetland Sheepdogs are predisposed to primary CNS HS, characterized by inflammatory CSF. High total nucleated cell count and the presence of neoplastic cells support the use of CSF analysis as a valuable diagnostic test. Prognosis for CNS HS is poor, but further evaluation of inflammatory mechanisms may provide novel therapeutic opportunities.
Keywords: canine; central nervous system; cerebrospinal fluid; neoplasia.
© 2020 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals, Inc. on behalf of the American College of Veterinary Internal Medicine.
Conflict of interest statement
Authors declare no conflict of interest.
Figures




References
-
- Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39:74‐83. - PubMed
-
- Moore PF. A review of histiocytic diseases of dogs and cats. Vet Pathol. 2014;51:167‐184. - PubMed
-
- Moore PF, Rosin A. Malignant histiocytosis of Bernese Mountain dogs. Vet Pathol. 1986;23:1‐10. - PubMed
-
- Padgett GA, Madewell BR, Keller ET, Jodar L, Packard M. Inheritance of histiocytosis in Bernese Mountain dogs. J Small Anim Pract. 1995;36:93‐98. - PubMed

