Ixekizumab improves secondary lesional signs, pain and sexual health in patients with moderate-to-severe genital psoriasis
- PMID: 31919919
- PMCID: PMC7318177
- DOI: 10.1111/jdv.16181
Ixekizumab improves secondary lesional signs, pain and sexual health in patients with moderate-to-severe genital psoriasis
Abstract
Background: Epithelial surface disruption in genital psoriatic lesions may manifest as erosions, fissures and/or ulcers, causing pain and significantly impacting a patient's sexual health.
Objective: To evaluate the impact of erosions, fissures and/or ulcers in genital psoriatic lesions on pain and sexual activity in patients with moderate-to-severe genital psoriasis (GenPs) and treatment responses to ixekizumab vs. placebo until Week 12.
Methods: This post hoc subgroup analysis of patients presenting with and without erosions, fissures and/or ulcers in genital lesions from a phase IIIb multicentre, randomized, double-blind, placebo-controlled study (IXORA-Q; NCT02718898) in 149 adults with moderate-to-severe GenPs treated with subcutaneous ixekizumab (80 mg every 2 weeks; n = 75) or placebo (n = 74) evaluated outcomes for clinician-rated GenPs severity (static Physician's Global Assessment of Genitalia; sPGA-G) and patient-reported genital pain and itch (Genital Psoriasis Symptoms Scale; GPSS) and sexual health (Genital Psoriasis Sexual Frequency Questionnaire; GenPs-SFQ).
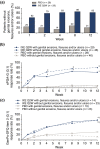
Results: At baseline, 38% (n = 57) of patients presented with genital erosions, fissures and/or ulcers independent of overall body surface area involvement (<10% or ≥10%). These signs were associated with higher scores for disease severity (sPGA-G) and pain (GPSS) but not sexual health (GenPs-SFQ). Complete resolution of these signs was observed in 62% of ixekizumab-treated patients (25% for placebo) at Week 1 and 83% (21% for placebo) at Week 12. Patients treated with ixekizumab reported significant improvements in pain, itch, disease severity and sexual health over 12 weeks compared to placebo and irrespective of the presence/absence of genital erosions, fissures and/or ulcers at baseline.
Conclusion: Ixekizumab led to rapid and sustained resolution of erosions, fissures and/or ulcers and significant improvements in GenPs severity, genital pain and sexual health. Ixekizumab may help to improve the well-being of patients with GenPs.
© 2020 Eli Lilly. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures
References
-
- Meeuwis KA, de Hullu JA, de Jager ME, Massuger LF, van de Kerkhof PC, van Rossum MM. Genital psoriasis: a questionnaire‐based survey on a concealed skin disease in the Netherlands. J Eur Acad Dermatol Venereol 2010; 24: 1425–1430. - PubMed
-
- Ryan C, Sadlier M, De Vol E et al Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol 2015; 72: 978–983. - PubMed
-
- Larsabal M, Ly S, Sbidian E et al GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol 2019; 180: 647–656. - PubMed
-
- Meeuwis KA, de Hullu JA, Massuger LF, van de Kerkhof PC, van Rossum MM. Genital psoriasis: a systematic literature review on this hidden skin disease. Acta Derm Venereol 2011; 91: 5–11. - PubMed
-
- Merola JF, Bleakman AP, Gottlieb AB et al The Static Physician's Global Assessment of Genitalia: a clinical outcome measure for the severity of genital psoriasis. J Drugs Dermatol 2017; 16: 793–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

