Long-Term Safety and Efficacy of Budesonide/Glycopyrrolate/Formoterol Fumarate Metered Dose Inhaler Formulated Using Co-Suspension Delivery Technology in Japanese Patients with COPD
- PMID: 31920296
- PMCID: PMC6934178
- DOI: 10.2147/COPD.S220861
Long-Term Safety and Efficacy of Budesonide/Glycopyrrolate/Formoterol Fumarate Metered Dose Inhaler Formulated Using Co-Suspension Delivery Technology in Japanese Patients with COPD
Abstract
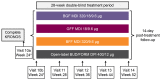
Background: Budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler (BGF MDI) is a triple fixed-dose combination for COPD. The long-term safety of triple therapy for COPD has not been investigated in Japanese patients. In this 28-week extension study (NCT03262012), we investigated the long-term safety and tolerability of BGF MDI in Japanese patients with moderate-to-very severe COPD who completed the 24-week Phase III randomized, double-blind, multicenter KRONOS study (NCT02497001).
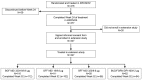
Materials and methods: Patients randomized to BGF MDI 320/18/9.6 μg, glycopyrrolate/formoterol fumarate (GFF) MDI 18/9.6 μg, budesonide/formoterol fumarate (BFF) MDI 320/9.6 μg, or budesonide/formoterol fumarate dry powder inhaler (BUD/FORM DPI) 400/12 μg twice-daily in KRONOS continued treatment for up to 28 additional weeks. Safety was evaluated over 52 weeks via adverse event (AE) monitoring, electrocardiograms, clinical laboratory testing, and vital sign measurements.
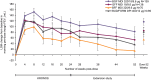
Results: The safety population included 416 patients who received BGF MDI (n=139), GFF MDI (n=138), BFF MDI (n=70), or BUD/FORM DPI (n=69). Treatment-emergent AE (TEAE) rates were similar across treatment groups (range: 82.6-82.9%). The most frequent TEAEs overall were nasopharyngitis (32.2%) and bronchitis (9.9%). The incidence of major adverse cardiovascular events was low across groups (range: 0.0-2.9%). Over 52 weeks, the incidence of confirmed pneumonia was 9.4% (BGF MDI), 3.6% (GFF MDI), 5.7% (BFF MDI), and 2.9% (BUD/FORM DPI); in the 28-week extension period, rates were comparable across groups (range: 2.9-5.7%). Six deaths were reported (0.7-2.2% per group); none were considered treatment-related. No clinically meaningful trends were observed in electrocardiograms, laboratory parameters, or vital signs over time in any of the treatment groups.
Conclusion: All treatments were well tolerated over 52 weeks, and the safety profile of BGF MDI was generally comparable to dual long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) and inhaled corticosteroid (ICS)/LABA therapies. These findings support the long-term tolerability of BGF MDI in Japanese patients with COPD.
Keywords: ICS/LAMA/LABA; Japan; co-suspension delivery technology; inhaled corticosteroid; long-acting muscarinic antagonist; long-acting β2-agonist.
© 2019 Ichinose et al.
Conflict of interest statement
MI reports personal fees from AstraZeneca, during the conduct of the study; and personal fees from Kyorin, Nippon Boehringer Ingelheim, and Novartis Pharma, outside of the submitted work. YI reports personal fees from GlaxoSmithKline, Nippon Boehringer Ingelheim, Nobelpharma, and Shionogi & Co. Ltd, outside of the submitted work. OH reports personal fees from AstraZeneca, Nippon Boehringer Ingelheim, and Novartis Pharma; and research funding from AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Nippon Boehringer Ingelheim, and Novartis Pharma. GTF reports grants, personal fees, and non-financial support from AstraZeneca during the conduct of the study; grants, personal fees, and non-financial support from AstraZeneca, Boehringer Ingelheim, Novartis, Pearl – a member of the AstraZeneca Group, and Sunovion; grants and personal fees from Theravance; and personal fees from Circassia, GlaxoSmithKline, Innoviva, Mylan, and Verona, outside of the submitted work. KFR reports personal fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi Pharmaceuticals, InterMune, Novartis, Sanofi, and Teva; and grants from the Ministry of Education and Science, Germany, outside of the submitted work. NH, HO, and MT are employees of AstraZeneca K.K., Japan. EB, SB, MA, CR, and PD are employees of AstraZeneca. KdA is a former employee of AstraZeneca. The authors report no other conflicts of interest in this work.
Figures
References
-
- Global Initiative for Chronic Obstructive Lung Disease Japan. Statistical data on COPD. 2017. Available from: http://www.gold-jac.jp/copd_facts_in_japan/. Accessed November14, 2018.
-
- Global Initiative for Chronic Obstructive Lung Disease. 2019 Report: global strategy for the diagnosis, management and prevention of COPD. 2019. Available from: https://goldcopd.org. Accessed June28, 2019. - PubMed
-
- Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi:10.1016/S2213-2600(18)30327-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

