Manganese Oxide Nanozymes Ameliorate Mechanical Allodynia in a Rat Model of Partial Sciatic Nerve-Transection Induced Neuropathic Pain
- PMID: 31920306
- PMCID: PMC6938959
- DOI: 10.2147/IJN.S225594
Manganese Oxide Nanozymes Ameliorate Mechanical Allodynia in a Rat Model of Partial Sciatic Nerve-Transection Induced Neuropathic Pain
Abstract
Background: Reactive oxygen species (ROS) induced oxidative stress is linked to numerous neurological diseases, including neuropathic pain. Natural ROS scavenging enzymes like superoxide dismutase (SOD) and catalase have been found to be efficient in alleviating neuropathic pain. However, their sensitivity towards extreme pH and a short half-life limit their efficacy in vivo. Manganese oxide nanoparticles (MONPs) are recently known to possess ROS scavenging properties. In this study, MONPs were examined for their therapeutic effect on neuropathic pain.
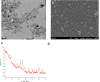
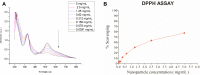
Methods: The MONPs were synthesized by a hydrothermal method. The synthesized MONPs were characterized by UV/Vis, TEM, SEM, FTIR, NTA and XRD. The biocompatibility of the nanoparticles is evaluated in neural cells using LDH assay. MONPs were evaluated for their antioxidant activity by DPPH assay. In addition, in vitro ROS scavenging properties were examined in bone marrow-derived macrophage (BMDM) cells using 2',7'-dichlorofluorescin diacetate (DCFDA) assay. To evaluate the in vivo efficacy of nanoparticles, neuropathic pain was induced in Wistar rats by partial sciatic nerve transection (PSNT). On post-transection days 14 to 18, rats were intrathecally injected with MONPs and paw withdrawal threshold was measured. The spinal cords were collected and processed for Western blotting and histological analysis.
Results: The synthesized MONPs were biocompatible and showed effective antioxidant activity against DPPH free radical scavenging. Further, the nanoparticles scavenged ROS efficiently in vitro in BMDM and their intrathecal administration significantly reduced mechanical allodynia as well as the expression of cyclooxygenase-2 (COX-2), an important mediator of chronic and inflammatory pain in the spinal dorsal horns of PSNT rats.
Conclusion: As ROS play a significant role in neuropathic pain, we expect that MONPs could be a promising tool for the treatment of various inflammatory diseases and might also serve as a potential nanocarrier for the delivery of analgesics.
Keywords: MONPs; allodynia; reactive oxygen species and neuropathic pain.
© 2019 Kuthati et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





Similar articles
-
The antioxidant N-(2-mercaptopropionyl)-glycine (tiopronin) attenuates expression of neuropathic allodynia and hyperalgesia.Naunyn Schmiedebergs Arch Pharmacol. 2021 Apr;394(4):603-617. doi: 10.1007/s00210-020-01995-y. Epub 2020 Oct 20. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33079239
-
The role of P2Y6 receptors in the maintenance of neuropathic pain and its improvement of oxidative stress in rats.J Cell Biochem. 2019 Oct;120(10):17123-17130. doi: 10.1002/jcb.28972. Epub 2019 May 20. J Cell Biochem. 2019. PMID: 31106899
-
Artichoke leaf hydroethanolic extract reduces neuropathic pain in a rat model of chronic constriction injury via attenuating the sciatic nerve oxidative stress.Arch Physiol Biochem. 2025 Apr;131(2):227-233. doi: 10.1080/13813455.2024.2406898. Epub 2024 Sep 25. Arch Physiol Biochem. 2025. PMID: 39320929
-
Nitroxidative Stress, Cell-Signaling Pathways, and Manganese Porphyrins: Therapeutic Potential in Neuropathic Pain.Int J Mol Sci. 2025 Feb 26;26(5):2050. doi: 10.3390/ijms26052050. Int J Mol Sci. 2025. PMID: 40076672 Free PMC article. Review.
-
Various gases for the treatment of neuropathic pain: mechanisms, current status, and future perspectives.Med Gas Res. 2025 Dec 1;15(4):488-495. doi: 10.4103/mgr.MEDGASRES-D-24-00161. Epub 2025 Apr 29. Med Gas Res. 2025. PMID: 40300884 Free PMC article. Review.
Cited by
-
Emerging role of macrophages in neuropathic pain.J Orthop Translat. 2025 Mar 18;51:227-241. doi: 10.1016/j.jot.2025.01.016. eCollection 2025 Mar. J Orthop Translat. 2025. PMID: 40177638 Free PMC article. Review.
-
Nanozymes-recent development and biomedical applications.J Nanobiotechnology. 2022 Feb 22;20(1):92. doi: 10.1186/s12951-022-01295-y. J Nanobiotechnology. 2022. PMID: 35193573 Free PMC article. Review.
-
Nanozymes in neuropathic pain: strategies bridging oxidative stress, mitochondrial repair, and neuroimmune modulation for targeted therapy.J Neuroinflammation. 2025 Jun 12;22(1):156. doi: 10.1186/s12974-025-03456-w. J Neuroinflammation. 2025. PMID: 40506712 Free PMC article. Review.
-
Potential of Nano-Antioxidants and Nanomedicine for Recovery from Neurological Disorders Linked to Long COVID Syndrome.Antioxidants (Basel). 2023 Feb 6;12(2):393. doi: 10.3390/antiox12020393. Antioxidants (Basel). 2023. PMID: 36829952 Free PMC article. Review.
-
Recent Advances in ROS-Scavenging Metallic Nanozymes for Anti-Inflammatory Diseases: A Review.Chonnam Med J. 2023 Jan;59(1):13-23. doi: 10.4068/cmj.2023.59.1.13. Epub 2023 Jan 25. Chonnam Med J. 2023. PMID: 36794252 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

