Potential Antiangiogenic Treatment Eligibility of Patients with Squamous Non-Small-Cell Lung Cancer: EPISQUAMAB Study (GFPC 2015-01)
- PMID: 31920391
- PMCID: PMC6938186
- DOI: 10.2147/CMAR.S219984
Potential Antiangiogenic Treatment Eligibility of Patients with Squamous Non-Small-Cell Lung Cancer: EPISQUAMAB Study (GFPC 2015-01)
Abstract
Background: Antiangiogenic agents have improved the prognosis of non-squamous non-small-cell lung cancers (NSCLCs), even though all the patients are not eligible to receive them because of counterindications linked to the tumor's characteristics or comorbidities. Much less information is available about the eligibility of patients with squamous non-small-cell lung cancers (SQ-NSCLCs) to receive antivascular endothelial growth-factor (VEGF) treatments, even though such molecules are being developed for this histology. This study was undertaken to determine the percentage of advanced SQ-NSCLC patients who would be eligible to receive an antiVEGF agent as second-line systemic therapy.
Methods: This observational, multicenter, prospective study evaluated advanced SQ-NSCLC patients' criteria for ineligibility to receive an antiVEGF during a multidisciplinary meeting to choose their standard second-line systemic therapy.
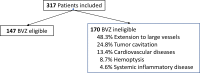
Results: Among the 317 patients included, 53.6% had at least one ineligibility criterion, and ~20% had at least two, with disease extension to large vessels (39.8%), tumor cavitation (20.5%), cardiovascular disease (11%) and/or hemoptysis (7.2%) being the most frequent. Patients with an ECOG performance score of 1/2 had more cardiovascular contraindications that those with scores of 0.
Conclusion: Almost half of the SQ-NSCLC patients included in this study would have been eligible to receive an antiVEGF agent. The development of these molecules for these indications should be encouraged.
Keywords: antiangiogenic treatments; lung cancer; squamous non-small cell.
© 2019 Vergnenègre et al.
Conflict of interest statement
A. Vergnenègre has received honoraria for consultancies and fees for medical conferences from MSD, Hoffman–La Roche, BMS, Pierre-Fabre Oncology, AstraZeneca, and Boehringer Ingelheim. Dr Olivier Bylicki reports personal fees from MSD, personal fees from ROCHE, personal fees from ASTRA-ZENECA, during the conduct of the study. The authors report no other conflicts of interest in this work.
Figures
References
-
- American Cancer Society. Cancer Facts & Figures. Vol. 1; 2019:219
LinkOut - more resources
Full Text Sources