Mechanisms of Immune Activation by c9orf72- Expansions in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
- PMID: 31920478
- PMCID: PMC6914852
- DOI: 10.3389/fnins.2019.01298
Mechanisms of Immune Activation by c9orf72- Expansions in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
Abstract
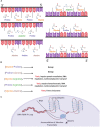
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are neurodegenerative disorders with overlapping pathomechanisms, neurobehavioral features, and genetic etiologies. Individuals diagnosed with either disorder exhibit symptoms within a clinical spectrum. Symptoms of ALS involve neuromusculature deficits, reflecting upper and lower motor neurodegeneration, while the primary clinical features of FTD are behavioral and cognitive impairments, reflecting frontotemporal lobar degeneration. An intronic G4C2 hexanucleotide repeat expansion (HRE) within the promoter region of chromosome 9 open reading frame 72 (C9orf72) is the predominant monogenic cause of both ALS and FTD. While the heightened risk to develop ALS/FTD in response to C9orf72 expansions is well-established, studies continue to define the precise mechanisms by which this mutation elicits neurodegeneration. Studies show that G4C2 expansions undergo repeat-associated non-ATG dependent (RAN) translation, producing dipeptide repeat proteins (DRPs) with varying toxicities. Accumulation of DRPs in neurons, in particular arginine containing DRPs, have neurotoxic effects by potently impairing nucleocytoplasmic transport, nucleotide metabolism, lysosomal processes, and cellular metabolic pathways. How these pathophysiological effects of C9orf72 expansions engage and elicit immune activity with additional neurobiological consequences is an important line of future investigations. Immunoreactive microglia and elevated levels of peripheral inflammatory cytokines noted in individuals with C9orf72 ALS/FTD provide evidence that persistent immune activation has a causative role in the progression of each disorder. This review highlights the current understanding of the cellular, proteomic and genetic substrates through which G4C2 HREs may elicit detrimental immune activity, facilitating region-specific neurodegeneration in C9orf72 mediated ALS/FTD. We in particular emphasize interactions between intracellular pathways induced by C9orf72 expansions and innate immune inflammasome complexes, intracellular receptors responsible for eliciting inflammation in response to cellular stress. A further understanding of the intricate, reciprocal relationship between the cellular and molecular pathologies resulting from C9orf72 HREs and immune activation may yield novel therapeutics for ALS/FTD, which currently have limited treatment strategies.
Keywords: C9orf72; TDP-43; amyotrophic lateral sclerosis (ALS); frontotemporal dementia (FTD); innate immunity; microglia; reactive oxygen species (ROS); therapeutics.
Copyright © 2019 Trageser, Smith, Herman, Ono and Pasinetti.
Figures
Similar articles
-
Inflammasome-Mediated Neuronal-Microglial Crosstalk: a Therapeutic Substrate for the Familial C9orf72 Variant of Frontotemporal Dementia/Amyotrophic Lateral Sclerosis.Mol Neurobiol. 2023 Jul;60(7):4004-4016. doi: 10.1007/s12035-023-03315-w. Epub 2023 Apr 3. Mol Neurobiol. 2023. PMID: 37010807
-
C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels.Autophagy. 2021 Nov;17(11):3306-3322. doi: 10.1080/15548627.2021.1872189. Epub 2021 Feb 26. Autophagy. 2021. PMID: 33632058 Free PMC article. Review.
-
Roadmap for C9ORF72 in Frontotemporal Dementia and Amyotrophic Lateral Sclerosis: Report on the C9ORF72 FTD/ALS Summit.Neurol Ther. 2023 Dec;12(6):1821-1843. doi: 10.1007/s40120-023-00548-8. Epub 2023 Oct 17. Neurol Ther. 2023. PMID: 37847372 Free PMC article.
-
Molecular Mechanisms of Neurodegeneration Related to C9orf72 Hexanucleotide Repeat Expansion.Behav Neurol. 2019 Jan 15;2019:2909168. doi: 10.1155/2019/2909168. eCollection 2019. Behav Neurol. 2019. PMID: 30774737 Free PMC article. Review.
-
C9orf72-Associated Dipeptide Repeat Expansions Perturb ER-Golgi Vesicular Trafficking, Inducing Golgi Fragmentation and ER Stress, in ALS/FTD.Mol Neurobiol. 2024 Dec;61(12):10318-10338. doi: 10.1007/s12035-024-04187-4. Epub 2024 May 9. Mol Neurobiol. 2024. PMID: 38722513 Free PMC article.
Cited by
-
Correlation between leukocyte phenotypes and prognosis of amyotrophic lateral sclerosis.Elife. 2022 Mar 15;11:e74065. doi: 10.7554/eLife.74065. Elife. 2022. PMID: 35287794 Free PMC article.
-
Plasma inflammation for predicting phenotypic conversion and clinical progression of autosomal dominant frontotemporal lobar degeneration.J Neurol Neurosurg Psychiatry. 2023 Jul;94(7):541-549. doi: 10.1136/jnnp-2022-330866. Epub 2023 Mar 28. J Neurol Neurosurg Psychiatry. 2023. PMID: 36977552 Free PMC article.
-
The Expanding Regulatory Mechanisms and Cellular Functions of Long Non-coding RNAs (lncRNAs) in Neuroinflammation.Mol Neurobiol. 2021 Jun;58(6):2916-2939. doi: 10.1007/s12035-020-02268-8. Epub 2021 Feb 8. Mol Neurobiol. 2021. PMID: 33555549 Review.
-
Stress granules: emerging players in neurodegenerative diseases.Transl Neurodegener. 2025 May 12;14(1):22. doi: 10.1186/s40035-025-00482-9. Transl Neurodegener. 2025. PMID: 40355949 Free PMC article. Review.
-
The role of neurofilament light in genetic frontotemporal lobar degeneration.Brain Commun. 2022 Nov 26;5(1):fcac310. doi: 10.1093/braincomms/fcac310. eCollection 2023. Brain Commun. 2022. PMID: 36694576 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

