High Salt Intake Lowers Behavioral Inhibition
- PMID: 31920580
- PMCID: PMC6923701
- DOI: 10.3389/fnbeh.2019.00271
High Salt Intake Lowers Behavioral Inhibition
Abstract
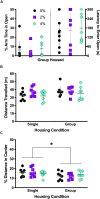
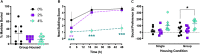
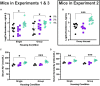
Stress-related neuropsychiatric (e.g., anxiety, depression) and cardiovascular diseases are frequently comorbid, though discerning the directionality of their association has been challenging. One of the most controllable risk factors for cardiovascular disease is salt intake. Though high salt intake is implicated in neuropsychiatric diseases, its direct neurobehavioral effects have seldom been explored. We reported that elevated salt intake in mice augments neuroinflammation, particularly after an acute stressor. Here, we explored how high salt consumption affected behavioral responses of mice to mildly arousing environmental and social tests, then assessed levels of the stress-related hormone corticosterone. Unexpectedly, anxiety-related behaviors in the elevated plus maze, open field, and marble burying test were unaffected by increased salt intake. However, nest building was diminished in mice consuming high salt, and voluntary social interaction was elevated, suggesting reduced engagement in ethologically-relevant behaviors that promote survival by attenuating threat exposure. Moreover, we observed significant positive correlations between social preference and subsequent corticosterone only in mice consuming increased salt, as well as negative correlations between open arm exploration in the elevated plus maze and corticosterone selectively in mice in the highest salt condition. Thus, heightened salt consumption reduces behavioral inhibition under relatively low-threat conditions, and enhances circulating corticosterone proportional to specific behavioral shifts. Considering the adverse health consequences of high salt intake, combined with evidence that increased salt consumption impairs inhibition of context-inappropriate behaviors, we suggest that prolonged high salt intake likely promulgates maladaptive behavioral and cardiovascular responses to perceived stressors that may explain some of the prevalent comorbidity between cardiovascular and neuropsychiatric diseases.
Keywords: anxiety; cardiovascular and neuropsychiatric comorbidity; corticosterone; salt loading; social behavior; stress.
Copyright © 2019 Gilman, George, Andrade, Mitchell, Toney and Daws.
Figures

References
-
- Abdoli A. (2017). Hypothesis: high salt intake as an inflammation amplifier might be involved in the pathogenesis of neuropsychiatric disorders. Clin Exp Neuroimmunology 8 146–157. 10.1111/cen3.12389 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

