Morphine Differentially Alters the Synaptic and Intrinsic Properties of D1R- and D2R-Expressing Medium Spiny Neurons in the Nucleus Accumbens
- PMID: 31920618
- PMCID: PMC6932971
- DOI: 10.3389/fnsyn.2019.00035
Morphine Differentially Alters the Synaptic and Intrinsic Properties of D1R- and D2R-Expressing Medium Spiny Neurons in the Nucleus Accumbens
Abstract
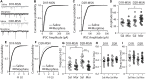
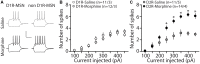
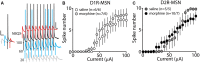
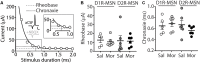
Exposure to opioids reshapes future reward and motivated behaviors partially by altering the functional output of medium spiny neurons (MSNs) in the nucleus accumbens shell. Here, we investigated how morphine, a highly addictive opioid, alters synaptic transmission and intrinsic excitability on dopamine D1-receptor (D1R) expressing and dopamine D2-receptor (D2R) expressing MSNs, the two main output neurons in the nucleus accumbens shell. Using whole-cell electrophysiology recordings, we show, that 24 h abstinence following repeated non-contingent administration of morphine (10 mg/kg, i.p.) in mice reduces the miniature excitatory postsynaptic current (mEPSC) frequency and miniature inhibitory postsynaptic current (mIPSC) frequency on D2R-MSNs, with concomitant increases in D2R-MSN intrinsic membrane excitability. We did not observe any changes in synaptic or intrinsic changes on D1R-MSNs. Last, in an attempt to determine the integrated effect of the synaptic and intrinsic alterations on the overall functional output of D2R-MSNs, we measured the input-output efficacy by measuring synaptically-driven action potential firing. We found that both D1R-MSN and D2R-MSN output was unchanged following morphine treatment.
Keywords: intrinsic excitability; morphine; neuronal activity; nucleus accumbens; opioid use disorder; synaptic transmission.
Copyright © 2019 McDevitt, Jonik and Graziane.
Figures


Similar articles
-
Fundamental Sex Differences in Cocaine-Induced Plasticity of Dopamine D1 Receptor- and D2 Receptor-Expressing Medium Spiny Neurons in the Mouse Nucleus Accumbens Shell.Biol Psychiatry Glob Open Sci. 2024 Feb 19;4(3):100295. doi: 10.1016/j.bpsgos.2024.100295. eCollection 2024 May. Biol Psychiatry Glob Open Sci. 2024. PMID: 38533248 Free PMC article.
-
Ventral Subiculum Inputs to Nucleus Accumbens Medial Shell Preferentially Innervate D2R Medium Spiny Neurons and Contain Calcium Permeable AMPARs.J Neurosci. 2023 Feb 15;43(7):1166-1177. doi: 10.1523/JNEUROSCI.1907-22.2022. Epub 2023 Jan 6. J Neurosci. 2023. PMID: 36609456 Free PMC article.
-
Sex differences in membrane properties and cellular excitability of dopamine D1 receptor-expressing neurons within the shell of the nucleus accumbens of pre- and mid-adolescent mice.Biol Sex Differ. 2024 Jul 13;15(1):54. doi: 10.1186/s13293-024-00631-1. Biol Sex Differ. 2024. PMID: 39003495 Free PMC article.
-
Sex Differences in Medium Spiny Neuron Excitability and Glutamatergic Synaptic Input: Heterogeneity Across Striatal Regions and Evidence for Estradiol-Dependent Sexual Differentiation.Front Endocrinol (Lausanne). 2018 Apr 18;9:173. doi: 10.3389/fendo.2018.00173. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29720962 Free PMC article. Review.
-
Reward signals downstream of dopamine D1 receptors.Nihon Arukoru Yakubutsu Igakkai Zasshi. 2016 Dec;51(6):371-381. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2016. PMID: 30461245 Review. English, Japanese.
Cited by
-
Adaptations in Nucleus Accumbens Neuron Subtypes Mediate Negative Affective Behaviors in Fentanyl Abstinence.Biol Psychiatry. 2023 Mar 15;93(6):489-501. doi: 10.1016/j.biopsych.2022.08.023. Epub 2022 Aug 30. Biol Psychiatry. 2023. PMID: 36435669 Free PMC article.
-
A Drd1-cre mouse line with nucleus accumbens gene dysregulation exhibits blunted fentanyl seeking.Neuropsychopharmacology. 2025 May 2. doi: 10.1038/s41386-025-02116-0. Online ahead of print. Neuropsychopharmacology. 2025. PMID: 40316698
-
Sustained fentanyl exposure inhibits neuronal activity in dissociated striatal neuronal-glial cocultures through actions independent of opioid receptors.J Neurophysiol. 2024 Sep 1;132(3):1056-1073. doi: 10.1152/jn.00444.2023. Epub 2024 Aug 7. J Neurophysiol. 2024. PMID: 39110896
-
Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens.Mol Psychiatry. 2022 Jan;27(1):669-686. doi: 10.1038/s41380-021-01112-2. Epub 2021 May 7. Mol Psychiatry. 2022. PMID: 33963288 Free PMC article. Review.
-
Dopamine D4 Receptor Is a Regulator of Morphine-Induced Plasticity in the Rat Dorsal Striatum.Cells. 2021 Dec 23;11(1):31. doi: 10.3390/cells11010031. Cells. 2021. PMID: 35011592 Free PMC article.
References
-
- Bertran-Gonzalez J., Bosch C., Maroteaux M., Matamales M., Hervé D., Valjent E., et al. . (2008). Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 28, 5671–5685. 10.1523/JNEUROSCI.1039-08.2008 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

