Incidence of Immune Checkpoint Inhibitor-Associated Diabetes: A Meta-Analysis of Randomized Controlled Studies
- PMID: 31920646
- PMCID: PMC6915045
- DOI: 10.3389/fphar.2019.01453
Incidence of Immune Checkpoint Inhibitor-Associated Diabetes: A Meta-Analysis of Randomized Controlled Studies
Abstract
Background: Immune checkpoint inhibitors (ICIs) are now an important option for more than 14 different cancers. Recent series case reports have described that ICIs are associated with new-onset diabetes in patients, yet the definitive risk is not available. We thus performed a meta-analysis of randomized controlled trials (RCTs) to assess the incidence and risk of developing new-onset diabetes following the use of ICIs. Methods: The PubMed, EMBASE, Cochrane Library databases, and ClinicalTrials.gov for RCTs were searched. Statistical analyses were performed using STATA 15 and R language. Fifty-two RCTs were included, and 12 did not report any events of ICI-associated diabetes. Results: A meta-analysis of 40 trials was performed, which reported at least one diabetes-related event among 24,596 patients. Although specific diabetes-related events were rare, compared with the placebo or other therapeutic strategies, the rates of serious hyperglycemia (OR 2.41, 95% CI 1.52 to 3.82), diabetes (3.54, 1.32 to 9.51), all-grade T1D (6.60, 2.51 to 17.30), and serious-grade T1D (6.50, 2.32 to 18.17) were increased with ICI drugs. Subgroup analysis according to the type of control, type of ICIs, and the combination mode suggested that ICIs plus conventional treatments significantly decreased the risks of diabetes and serious-grade hyperglycemia. There was little heterogeneity across the studies in all results except hyperglycemic events, which in part was attributable to data from everolimus-based control group. Conclusions: New-onset diabetes is uncommon with ICIs but the risk is increased compared with placebo or another therapeutic strategy. However, more studies are warranted to substantiate these findings across ICIs.
Keywords: diabetes; hyperglycemia; immune checkpoint inhibitors; meta-analysis; safety outcomes.
Copyright © 2019 Lu, Yang, Liang, Meng, Zhao and Zhang.
Figures
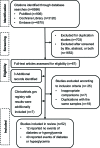
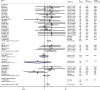
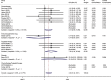

Comment in
-
Nebenwirkung Diabetes.MMW Fortschr Med. 2022 Apr;164(8):12-14. doi: 10.1007/s15006-022-1069-7. MMW Fortschr Med. 2022. PMID: 35449255 German. No abstract available.
References
-
- AstraZeneca (2015). “A Global Study to Assess the Effects of MEDI4736 (Durvalumab), Given as Monotherapy or in Combination With Tremelimumab Determined by PD-L1 Expression Versus Standard of Care in Patients With Locally Advanced or Metastatic Non Small Cell Lung Cancer”. https://ClinicalTrials.gov/show/NCT02352948).
-
- Barlesi F., Vansteenkiste J., Spigel D., Ishii H., Garassino M., de Marinis F., et al. (2018). Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 19 (11), 1468–1479. 10.1016/S1470-2045(18)30673-9 - DOI - PubMed
-
- Beer T. M., Kwon E. D., Drake C. G., Fizazi K., Logothetis C., Gravis G., et al. (2017). Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients With metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 35 (1), 40–47. 10.1200/JCO.2016.69.1584 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

