Effectiveness of Adalimumab for the Treatment of Psoriatic Arthritis: An Italian Real-Life Retrospective Study
- PMID: 31920675
- PMCID: PMC6923751
- DOI: 10.3389/fphar.2019.01497
Effectiveness of Adalimumab for the Treatment of Psoriatic Arthritis: An Italian Real-Life Retrospective Study
Abstract
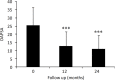
Background: Few studies have evaluated the effectiveness of adalimumab in the real-life setting in psoriatic arthritis (PsA). Objective: To evaluate the 2-year retention rate of adalimumab in PsA patients. Potential baseline parameters influencing persistence on treatment were also evaluated. Methods: PsA patients from 16 Italian Rheumatology Units treated with adalimumab as first- or second-line biological therapy were retrospectively evaluated. Adalimumab retention rate was evaluated at 12 and 24 months. Logistic regression was used to evaluate the association between predictor variables and adalimumab retention rate. Results: From 424 patients (53.5% male, aged 48.3 ± 12.8 years) who started treatment with adalimumab, 367 (86.6%) maintained treatment for 12 months and 313 (73.8%) for 2 years. At 24-months, Disease Activity in PsA (DAPSA) remission (defined as ≤4) and Low Disease Activity (LDA) (≤14) were achieved in 22.8% and 44.4% of patients, respectively. Adalimumab treatment significantly decreased the number of tender (7.0 ± 5.7 at baseline vs. 2.3 ± 3.5 at 24 months, p < 0.001) and swollen joints (2.7 ± 2.8 at baseline vs. 0.4 ± 0.9 at 24 months, p < 0.001), DAPSA (25.5 ± 10.9 at baseline vs. 11.0 ± 8.4 at 24 months, p < 0.001), PASI (5.3 ± 5.7 at baseline vs. 2.7 ± 2.8 at 24 months, p < 0.001) and CRP (3.8 ± 6.3 at baseline vs. 1.2 ± 1.7 at 24 months, p < 0.001). Among a range of laboratory and clinical variables, only female gender was associated with improved adalimumab persistence at 24 months (OR: 1.98, 95% CI: 1.2-3.2, p = 0.005). Conclusions: Independent of a range of predictor variables, adalimumab was shown to be effective, while maintaining a high retention rate after 2 years in PsA patients.
Keywords: adalimumab; biological drugs; psoriatic arthritis; real-life; retention rate.
Copyright © 2019 D'Angelo, Cantini, Ramonda, Cantarini, Carletto, Chimenti, Delle Sedie, Foti, Gerli, Lomater, Lubrano, Marchesoni, Zabotti, Salvarani, Scrivo, Scarpa, Tramontano, Nannini, Lorenzin, Fabbroni, Martinis, Perricone, Carli, Visalli, Rovera, Perrotta, Quartuccio, Altobelli, Costa, Niccoli, Ortolan and Caso.
Figures

References
-
- Aaltonen K., Heinonen A., Joensuu J., Parmanne P., Karjalainen A., Varjolahti-Lehtinen T., et al. (2017). Effectiveness and drug survival of TNF-inhibitors in the treatment of psoriatic arthritis: a prospective cohort study. Semin. Arthritis Rheumatol. 46, 732–739. 10.1016/j.semarthrit.2016.09.005 - DOI - PubMed
-
- Burmester G. R., Panaccione R., Gordon K. B., McIlraith M. J., Lacerda A. P. M. (2013). Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann. Rheumatol. Dis. 72, 517–524. 10.1136/annrheumdis-2011-201244 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

