VPS35-Based Approach: A Potential Innovative Treatment in Parkinson's Disease
- PMID: 31920908
- PMCID: PMC6928206
- DOI: 10.3389/fneur.2019.01272
VPS35-Based Approach: A Potential Innovative Treatment in Parkinson's Disease
Abstract
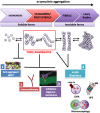
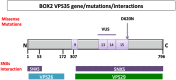
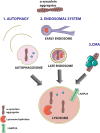
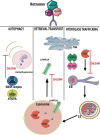
Several symptomatic treatments for Parkinson's disease (PD) are currently available. Still, the challenge today is to find a therapy that might reduce degeneration and slow down disease progression. The identification of pathogenic mutations in familial Parkinsonism (fPD) associated to the monogenic forms of PD provided pathophysiological insights and highlighted novel targets for therapeutic intervention. Mutations in the VPS35 gene have been associated with autosomal dominant fPD and a clinical phenotype indistinguishable from idiopathic PD. Although VPS35 mutations are relatively rare causes of PD, their study may help understanding specific cellular and molecular alterations that lead to accumulation α-synuclein in neurons of PD patients. Interacting with such mechanisms may provide innovative therapeutic approaches. We review here the evidence on the physiological role of VPS35 as a key intracellular trafficking protein controlling relevant neuronal functions. We further analyze VPS35 activity on α-synuclein degradation pathways that control the equilibrium between α-synuclein clearance and accumulation. Finally, we highlight the strategies for increasing VPS35 levels as a potential tool to treat PD.
Keywords: Parkinson's disease; alpha-synucein; amyloid protein A (AA); endosomal trafficking; retromer complex; therapeutic targets.
Copyright © 2019 Eleuteri and Albanese.
Figures


Similar articles
-
VPS35 and α-Synuclein fail to interact to modulate neurodegeneration in rodent models of Parkinson's disease.Mol Neurodegener. 2023 Aug 4;18(1):51. doi: 10.1186/s13024-023-00641-4. Mol Neurodegener. 2023. PMID: 37542299 Free PMC article.
-
VPS35 and the mitochondria: Connecting the dots in Parkinson's disease pathophysiology.Neurobiol Dis. 2020 Nov;145:105056. doi: 10.1016/j.nbd.2020.105056. Epub 2020 Aug 24. Neurobiol Dis. 2020. PMID: 32853677 Review.
-
VPS35 in Dopamine Neurons Is Required for Endosome-to-Golgi Retrieval of Lamp2a, a Receptor of Chaperone-Mediated Autophagy That Is Critical for α-Synuclein Degradation and Prevention of Pathogenesis of Parkinson's Disease.J Neurosci. 2015 Jul 22;35(29):10613-28. doi: 10.1523/JNEUROSCI.0042-15.2015. J Neurosci. 2015. PMID: 26203154 Free PMC article.
-
VPS35, the Retromer Complex and Parkinson's Disease.J Parkinsons Dis. 2017;7(2):219-233. doi: 10.3233/JPD-161020. J Parkinsons Dis. 2017. PMID: 28222538 Free PMC article. Review.
-
Mechanisms of VPS35-Mediated Neurodegeneration in Parkinson's Disease.Int Rev Mov Disord. 2021;2:221-244. doi: 10.1016/bs.irmvd.2021.08.005. Epub 2021 Sep 30. Int Rev Mov Disord. 2021. PMID: 35497708 Free PMC article.
Cited by
-
The Role of Vesicle Trafficking Defects in the Pathogenesis of Prion and Prion-Like Disorders.Int J Mol Sci. 2020 Sep 23;21(19):7016. doi: 10.3390/ijms21197016. Int J Mol Sci. 2020. PMID: 32977678 Free PMC article. Review.
-
Mitochondrial membrane proteins and VPS35 orchestrate selective removal of mtDNA.Nat Commun. 2022 Nov 7;13(1):6704. doi: 10.1038/s41467-022-34205-9. Nat Commun. 2022. PMID: 36344526 Free PMC article.
-
Polarized α-synuclein trafficking and transcytosis across brain endothelial cells via Rab7-decorated carriers.Fluids Barriers CNS. 2022 May 30;19(1):37. doi: 10.1186/s12987-022-00334-y. Fluids Barriers CNS. 2022. PMID: 35637478 Free PMC article.
-
Dopaminergic Axons: Key Recitalists in Parkinson's Disease.Neurochem Res. 2022 Feb;47(2):234-248. doi: 10.1007/s11064-021-03464-1. Epub 2021 Oct 12. Neurochem Res. 2022. PMID: 34637100 Review.
-
VPS35-Retromer: Multifunctional Roles in Various Biological Processes - A Focus on Neurodegenerative Diseases and Cancer.J Inflamm Res. 2025 Apr 3;18:4665-4680. doi: 10.2147/JIR.S510768. eCollection 2025. J Inflamm Res. 2025. PMID: 40195959 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

