Diverse Expression of Antimicrobial Activities Against Bacterial Vaginosis and Urinary Tract Infection Pathogens by Cervicovaginal Microbiota Strains of Lactobacillus gasseri and Lactobacillus crispatus
- PMID: 31921075
- PMCID: PMC6933176
- DOI: 10.3389/fmicb.2019.02900
Diverse Expression of Antimicrobial Activities Against Bacterial Vaginosis and Urinary Tract Infection Pathogens by Cervicovaginal Microbiota Strains of Lactobacillus gasseri and Lactobacillus crispatus
Abstract
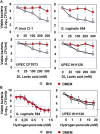
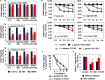
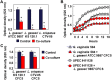
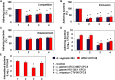
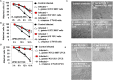
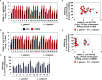
We aimed to analyze the strain-by-strain expression of a large panel of antimicrobial activities counteracting the virulence mechanisms of bacterial vaginosis-associated Prevotella bivia CI-1 and Gardnerella vaginalis 594, pyelonephritis-associated Escherichia coli CFT073, and recurrent cystitis- and preterm labor-associated IH11128 E. coli by Lactobacillus gasseri and Lactobacillus crispatus clinical strains, and L. gasseri ATCC 9857 and KS 120.1, and L. crispatus CTV-05 strains isolated from the cervicovaginal microbiota of healthy women. All L. gasseri and L. crispatus strains exerted antimicrobial activity by secreted lactic acid, which killed the microbial pathogens by direct contact. Potent bactericidal activity was exerted by a very limited number of resident L. gasseri and L. crispatus strains showing the specific ability to a strain to produce and release antibiotic-like compounds. These compounds eradicated the microbial pathogens pre-associated with the surface of cervix epithelial cells, providing efficient protection of the cells against the deleterious effects triggered by toxin-producing G. vaginalis and uropathogenic E. coli. Furthermore, these compounds crossed the cell membrane to kill the pre-internalized microbial pathogens. In addition, all L. gasseri and L. crispatus cells exhibited another non-strain specific activity which inhibited the association of microbial pathogens with cervix epithelial cells with varying efficiency, partially protecting the cells against lysis and detachment triggered by toxin-producing G. vaginalis and uropathogenic E. coli. Our results provide evidence of strain-level specificity for certain antimicrobial properties among cervicovaginal L. gasseri and L. crispatus strains, indicating that the presence of a particular species in the vaginal microbiota is not sufficient to determine its benefit to the host. A full repertory of antimicrobial properties should be evaluated in choosing vaginal microbiota-associated Lactobacillus isolates for the development of live biotherapeutic strategies.
Keywords: Lactobacillus crispatus; Lactobacillus gasseri; antimicrobial; bacterial vaginosis; cervicovaginal microbiota; urinary tract infections.
Copyright © 2019 Atassi, Pho Viet Ahn and Lievin-Le Moal.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

