RPS27, a sORF-Encoded Polypeptide, Functions Antivirally by Activating the NF-κB Pathway and Interacting With Viral Envelope Proteins in Shrimp
- PMID: 31921103
- PMCID: PMC6928191
- DOI: 10.3389/fimmu.2019.02763
RPS27, a sORF-Encoded Polypeptide, Functions Antivirally by Activating the NF-κB Pathway and Interacting With Viral Envelope Proteins in Shrimp
Abstract
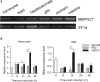
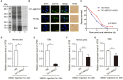
A small open reading frame (smORF) or short open reading frame (sORF) encodes a polypeptide of <100 amino acids in eukaryotes (50 amino acids in prokaryotes). Studies have shown that several sORF-encoded peptides (SEPs) have important physiological functions in different organisms. Many ribosomal proteins belonging to SEPs play important roles in several cellular processes, such as DNA damage repair and apoptosis. Several studies have implicated SEPs in response to infection and innate immunity, but the mechanisms have been unclear for most of them. In this study, we identified a sORF-encoded ribosomal protein S27 (RPS27) in Marsupenaeus japonicus. The expression of MjRPS27 was significantly upregulated in shrimp infected with white spot syndrome virus (WSSV). After knockdown of MjRPS27 by RNA interference, WSSV replication increased significantly. Conversely, after MjRPS27 overexpression, WSSV replication decreased in shrimp and the survival rate of the shrimp increased significantly. These results suggested that MjRPS27 inhibited viral replication. Further study showed that, after MjRPS27 knockdown, the mRNA expression level of MjDorsal, MjRelish, and antimicrobial peptides (AMPs) decreased, and the nuclear translocation of MjDorsal and MjRelish into the nucleus also decreased. These findings indicated that MjRPS27 might activate the NF-κB pathway and regulate the expression of AMPs in shrimp after WSSV challenge, thereby inhibiting viral replication. We also found that MjRPS27 interacted with WSSV's envelope proteins, including VP19, VP24, and VP28, suggesting that MjRPS27 may inhibit WSSV proliferation by preventing virion assembly in shrimp. This study was the first to elucidate the function of the ribosomal protein MjRPS27 in the antiviral immunity of shrimp.
Keywords: Relish; antimicrobial peptides; dorsal; kuruma shrimp; sORF encoded polypeptides; short open reading frame (sORF); white spot syndrome virus.
Copyright © 2019 Diao, Li, Xu, Zhao and Wang.
Figures

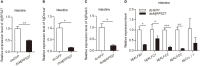



Similar articles
-
Infection and intracellular transport of white spot syndrome virus require the ESCRT machinery in shrimp.J Virol. 2024 Jul 23;98(7):e0043324. doi: 10.1128/jvi.00433-24. Epub 2024 Jun 18. J Virol. 2024. PMID: 38888346 Free PMC article.
-
Interaction of the Small GTPase Cdc42 with Arginine Kinase Restricts White Spot Syndrome Virus in Shrimp.J Virol. 2017 Feb 14;91(5):e01916-16. doi: 10.1128/JVI.01916-16. Print 2017 Mar 1. J Virol. 2017. PMID: 28031362 Free PMC article.
-
The sORF-Encoded Peptides, ATP Synthase Subunits, Facilitate WSSV Duplication in Shrimp.Viruses. 2022 Nov 4;14(11):2449. doi: 10.3390/v14112449. Viruses. 2022. PMID: 36366547 Free PMC article.
-
The Two NF-κB Pathways Regulating Bacterial and WSSV Infection of Shrimp.Front Immunol. 2019 Jul 30;10:1785. doi: 10.3389/fimmu.2019.01785. eCollection 2019. Front Immunol. 2019. PMID: 31417561 Free PMC article. Review.
-
Recent insights into host-pathogen interaction in white spot syndrome virus infected penaeid shrimp.J Fish Dis. 2015 Jul;38(7):599-612. doi: 10.1111/jfd.12279. Epub 2014 Jun 23. J Fish Dis. 2015. PMID: 24953507 Review.
Cited by
-
Infection and intracellular transport of white spot syndrome virus require the ESCRT machinery in shrimp.J Virol. 2024 Jul 23;98(7):e0043324. doi: 10.1128/jvi.00433-24. Epub 2024 Jun 18. J Virol. 2024. PMID: 38888346 Free PMC article.
-
Nitric Oxide Synthase Regulates Gut Microbiota Homeostasis by ERK-NF-κB Pathway in Shrimp.Front Immunol. 2021 Dec 3;12:778098. doi: 10.3389/fimmu.2021.778098. eCollection 2021. Front Immunol. 2021. PMID: 34925352 Free PMC article.
-
Localization and Functional Roles of Components of the Translation Apparatus in the Eukaryotic Cell Nucleus.Cells. 2021 Nov 19;10(11):3239. doi: 10.3390/cells10113239. Cells. 2021. PMID: 34831461 Free PMC article. Review.
-
Design of Three Residues Peptides against SARS-CoV-2 Infection.Viruses. 2022 Sep 22;14(10):2103. doi: 10.3390/v14102103. Viruses. 2022. PMID: 36298659 Free PMC article.
-
Peripheral blood transcriptomic analysis identifies potential inflammation and immune signatures for central retinal artery occlusion.Sci Rep. 2024 Mar 28;14(1):7398. doi: 10.1038/s41598-024-57052-8. Sci Rep. 2024. PMID: 38548806 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

