Cancer Neoepitopes for Immunotherapy: Discordance Between Tumor-Infiltrating T Cell Reactivity and Tumor MHC Peptidome Display
- PMID: 31921104
- PMCID: PMC6918724
- DOI: 10.3389/fimmu.2019.02766
Cancer Neoepitopes for Immunotherapy: Discordance Between Tumor-Infiltrating T Cell Reactivity and Tumor MHC Peptidome Display
Abstract
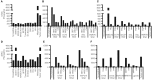
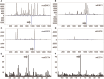
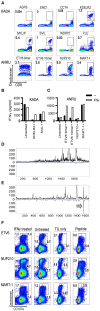
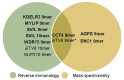
Tumor-infiltrating lymphocytes (TIL) are considered enriched for T cells recognizing shared tumor antigens or mutation-derived neoepitopes. We performed exome sequencing and HLA-A*02:01 epitope prediction from tumor cell lines from two HLA-A2-positive melanoma patients whose TIL displayed strong tumor reactivity. The potential neoepitopes were screened for recognition using autologous TIL by immunological assays and presentation on tumor major histocompatibility complex class I (MHC-I) molecules by Poisson detection mass spectrometry (MS). TIL from the patients recognized 5/181 and 3/49 of the predicted neoepitopes, respectively. MS screening detected 3/181 neoepitopes on tumor MHC-I from the first patient but only one was also among those recognized by TIL. Consequently, TIL enriched for neoepitope specificity failed to recognize tumor cells, despite being activated by peptides. For the second patient, only after IFN-γ treatment of the tumor cells was one of 49 predicted neoepitopes detected by MS, and this coincided with recognition by TIL sorted for the same specificity. Importantly, specific T cells could be expanded from patient and donor peripheral blood mononuclear cells (PBMC) for all neoepitopes recognized by TIL and/or detected on tumor MHC-I. In summary, stimulating the appropriate inflammatory environment within tumors may promote neoepitope MHC presentation while expanding T cells in blood may circumvent lack of specific TIL. The discordance in detection between physical and functional methods revealed here can be rationalized and used to improve neoantigen-targeted T cell immunotherapy.
Keywords: Immune peptidome; TIL; immunotherapy; mass spectrometry; neoepitopes; tumor.
Copyright © 2019 Wickström, Lövgren, Volkmar, Reinhold, Duke-Cohan, Hartmann, Rebmann, Mueller, Melief, Maas, Ligtenberg, Hansson, Offringa, Seliger, Poschke, Reinherz and Kiessling.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

