Understanding and Modulating Immunity With Cell Reprogramming
- PMID: 31921109
- PMCID: PMC6917620
- DOI: 10.3389/fimmu.2019.02809
Understanding and Modulating Immunity With Cell Reprogramming
Abstract
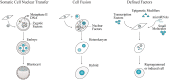
Cell reprogramming concepts have been classically developed in the fields of developmental and stem cell biology and are currently being explored for regenerative medicine, given its potential to generate desired cell types for replacement therapy. Cell fate can be experimentally reversed or modified by enforced expression of lineage specific transcription factors leading to pluripotency or attainment of another somatic cell type identity. The possibility to reprogram fibroblasts into induced dendritic cells (DC) competent for antigen presentation creates a paradigm shift for understanding and modulating the immune system with direct cell reprogramming. PU.1, IRF8, and BATF3 were identified as sufficient and necessary to impose DC fate in unrelated cell types, taking advantage of Clec9a, a C-type lectin receptor with restricted expression in conventional DC type 1. The identification of such minimal gene regulatory networks helps to elucidate the molecular mechanisms governing development and lineage heterogeneity along the hematopoietic hierarchy. Furthermore, the generation of patient-tailored reprogrammed immune cells provides new and exciting tools for the expanding field of cancer immunotherapy. Here, we summarize cell reprogramming concepts and experimental approaches, review current knowledge at the intersection of cell reprogramming with hematopoiesis, and propose how cell fate engineering can be merged to immunology, opening new opportunities to understand the immune system in health and disease.
Keywords: antigen presentation; cancer immunotherapy; cell fate reprogramming; dendritic cell; hematopoiesis; regenerative medicine; transcription factor; transdifferentiation.
Copyright © 2019 Pires, Rosa, Kurochkin and Pereira.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

