WNT Signaling in Tumors: The Way to Evade Drugs and Immunity
- PMID: 31921125
- PMCID: PMC6934036
- DOI: 10.3389/fimmu.2019.02854
WNT Signaling in Tumors: The Way to Evade Drugs and Immunity
Abstract
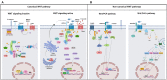
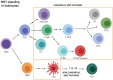
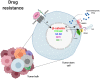
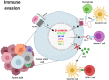
WNT/β-catenin signaling is involved in many physiological processes. Its implication in embryonic development, cell migration, and polarization has been shown. Nevertheless, alterations in this signaling have also been related with pathological events such as sustaining and proliferating the cancer stem cell (CSC) subset present in the tumor bulk. Related with this, WNT signaling has been associated with the maintenance, expansion, and epithelial-mesenchymal transition of stem cells, and furthermore with two distinctive features of this tumor population: therapeutic resistance (MDR, multidrug resistance) and immune escape. These mechanisms are developed and maintained by WNT activation through the transcriptional control of the genes involved in such processes. This review focuses on the description of the best known WNT pathways and the molecules involved in them. Special attention is given to the WNT cascade proteins deregulated in tumors, which have a decisive role in tumor survival. Some of these proteins function as extrusion pumps that, in the course of chemotherapy, expel the drugs from the cells; others help the tumoral cells hide from the immune effector mechanisms. Among the WNT targets involved in drug resistance, the drug extrusion pump MDR-1 (P-GP, ABCB1) and the cell adhesion molecules from the CD44 family are highlighted. The chemokine CCL4 and the immune checkpoint proteins CD47 and PD-L1 are included in the list of WNT target molecules with a role in immunity escape. This pathway should be a main target in cancer therapy as WNT signaling activation is essential for tumor progression and survival, even in the presence of the anti-tumoral immune response and/or antineoplastic drugs. The appropriate design and combination of anti-tumoral strategies, based on the modulation of WNT mediators and/or protein targets, could negatively affect the growth of tumoral cells, improving the efficacy of these types of therapies.
Keywords: ABCB1; CD47; PD-L1; WNT; cancer; immunity escape; multidrug resistance (MDR); β-catenin.
Copyright © 2019 Martin-Orozco, Sanchez-Fernandez, Ortiz-Parra and Ayala-San Nicolas.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

