Coexpression of CCR7 and CXCR4 During B Cell Development Controls CXCR4 Responsiveness and Bone Marrow Homing
- PMID: 31921208
- PMCID: PMC6930800
- DOI: 10.3389/fimmu.2019.02970
Coexpression of CCR7 and CXCR4 During B Cell Development Controls CXCR4 Responsiveness and Bone Marrow Homing
Abstract
The CXCL12-CXCR4 axis plays a key role in the retention of stem cells and progenitors in dedicated bone marrow niches. It is well-known that CXCR4 responsiveness in B lymphocytes decreases dramatically during the final stages of their development in the bone marrow. However, the molecular mechanism underlying this regulation and whether it plays a role in B-cell homeostasis remain unknown. In the present study, we show that the differentiation of pre-B cells into immature and mature B cells is accompanied by modifications to the relative expression of chemokine receptors, with a two-fold downregulation of CXCR4 and upregulation of CCR7. We demonstrate that expression of CCR7 in B cells is involved in the selective inactivation of CXCR4, and that mature B cells from CCR7-/- mice display higher responsiveness to CXCL12 and improved retention in the bone marrow. We also provide molecular evidence supporting a model in which upregulation of CCR7 favors the formation of CXCR4-CCR7 heteromers, wherein CXCR4 is selectively impaired in its ability to activate certain G-protein complexes. Collectively, our results demonstrate that CCR7 behaves as a novel selective endogenous allosteric modulator of CXCR4.
Keywords: B cells; CCR7; CXCR4; homing; lymphopoiesis.
Copyright © 2019 Mcheik, Van Eeckhout, De Poorter, Galés, Parmentier and Springael.
Figures




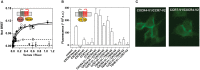
 ) or CCR7-hRLuc (♢) as donor. The Net BRET corresponds to the BRET measured between the two partners minus the BRET measured in cells expressing CXCR4-hRLuc or CCR7-hRLuc only. Data represent mean values ± SEM (n = 3). (B) CCR7 interacts with CXCR4 in a fluorescence complementation assay. HEK293T cells were transfected with CXCR4-V1, CXCR4-V2, CCR7-V1, and CCR7-V2 constructs, alone or as two by two combinations, and the fluorescence emission was recorded. As controls, TSHR-V1 and TSHR-V2 were cotransfected with the various CXCR4 and CCR7 constructs. Data represent mean values ± SEM (n = 3). (C) CCR7 interacts with CXCR4 at the plasma membrane. HEK293T cells were cotransfected with CXCR4-V1 and CCR7-V2 or CXCR4-V2 and CCR7-V1, and fluorescence was monitored by using fluorescent microscopy.
) or CCR7-hRLuc (♢) as donor. The Net BRET corresponds to the BRET measured between the two partners minus the BRET measured in cells expressing CXCR4-hRLuc or CCR7-hRLuc only. Data represent mean values ± SEM (n = 3). (B) CCR7 interacts with CXCR4 in a fluorescence complementation assay. HEK293T cells were transfected with CXCR4-V1, CXCR4-V2, CCR7-V1, and CCR7-V2 constructs, alone or as two by two combinations, and the fluorescence emission was recorded. As controls, TSHR-V1 and TSHR-V2 were cotransfected with the various CXCR4 and CCR7 constructs. Data represent mean values ± SEM (n = 3). (C) CCR7 interacts with CXCR4 at the plasma membrane. HEK293T cells were cotransfected with CXCR4-V1 and CCR7-V2 or CXCR4-V2 and CCR7-V1, and fluorescence was monitored by using fluorescent microscopy.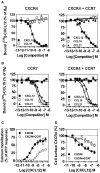
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

