Validation of a Novel, Sensitive, and Specific Urine-Based Test for Recurrence Surveillance of Patients With Non-Muscle-Invasive Bladder Cancer in a Comprehensive Multicenter Study
- PMID: 31921291
- PMCID: PMC6930177
- DOI: 10.3389/fgene.2019.01237
Validation of a Novel, Sensitive, and Specific Urine-Based Test for Recurrence Surveillance of Patients With Non-Muscle-Invasive Bladder Cancer in a Comprehensive Multicenter Study
Abstract
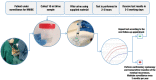
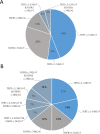
Bladder cancer (BC), the most frequent malignancy of the urinary system, is ranked the sixth most prevalent cancer worldwide. Of all newly diagnosed patients with BC, 70-75% will present disease confined to the mucosa or submucosa, the non-muscle-invasive BC (NMIBC) subtype. Of those, approximately 70% will recur after transurethral resection (TUR). Due to high rate of recurrence, patients are submitted to an intensive follow-up program maintained throughout many years, or even throughout life, resulting in an expensive follow-up, with cystoscopy being the most cost-effective procedure for NMIBC screening. Currently, the gold standard procedure for detection and follow-up of NMIBC is based on the association of cystoscopy and urine cytology. As cystoscopy is a very invasive approach, over the years, many different noninvasive assays (both based in serum and urine samples) have been developed in order to search genetic and protein alterations related to the development, progression, and recurrence of BC. TERT promoter mutations and FGFR3 hotspot mutations are the most frequent somatic alterations in BC and constitute the most reliable biomarkers for BC. Based on these, we developed an ultra-sensitive, urine-based assay called Uromonitor®, capable of detecting trace amounts of TERT promoter (c.1-124C > T and c.1-146C > T) and FGFR3 (p.R248C and p.S249C) hotspot mutations, in tumor cells exfoliated to urine samples. Cells present in urine were concentrated by the filtration of urine through filters where tumor cells are trapped and stored until analysis, presenting long-term stability. Detection of the alterations was achieved through a custom-made, robust, and highly sensitive multiplex competitive allele-specific discrimination PCR allowing clear interpretation of results. In this study, we validate a test for NMIBC recurrence detection, using for technical validation a total of 331 urine samples and 41 formalin-fixed paraffin-embedded tissues of the primary tumor and recurrence lesions from a large cluster of urology centers. In the clinical validation, we used 185 samples to assess sensitivity/specificity in the detection of NMIBC recurrence vs. cystoscopy/cytology and in a smaller cohort its potential as a primary diagnostic tool for NMIBC. Our results show this test to be highly sensitive (73.5%) and specific (93.2%) in detecting recurrence of BC in patients under surveillance of NMIBC.
Keywords: FGFR3 mutation; TERT promoter mutation; Uromonitor®; non-muscle invasive bladder cancer; urinary test.
Copyright © 2019 Batista, Vinagre, Prazeres, Sampaio, Peralta, Conceição, Sismeiro, Leão, Gomes, Furriel, Oliveira, Torres, Eufrásio, Azinhais, Almeida, Gonzalez, Bidovanets, Ecke, Stinjs, Pascual, Abdelmalek, Villafruela, Beardo-Villar, Fidalgo, Öztürk, Gonzalez-Enguita, Monzo, Lopes, Álvarez-Maestro, Servan, De La Cruz, Perez, Máximo and Soares.
Figures





References
-
- Allory Y., Beukers W., Sagrera A., Flandez M., Marques M., Marquez M., et al. (2014). Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur. Urol. 65 (2), 360–366. 10.1016/j.eururo.2013.08.052 - DOI - PubMed
-
- Babjuk M., Burger M., Compérat E., Gontero P., Mostafid A. H., Palou J., et al. (2018). EUA Guidelines on Non-muscle-invasive Bladder Cancer. Edn. presented at the EAU Annual Congress Copenhagen 2018.
LinkOut - more resources
Full Text Sources
Research Materials

