Chemogenetic interactions in human cancer cells
- PMID: 31921397
- PMCID: PMC6945272
- DOI: 10.1016/j.csbj.2019.09.006
Chemogenetic interactions in human cancer cells
Abstract
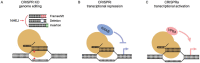
Chemogenetic profiling enables the identification of genes that enhance or suppress the phenotypic effect of chemical compounds. Using this approach in cancer therapies could improve our ability to predict the response of specific tumor genotypes to chemotherapeutic agents, thus accelerating the development of personalized drug therapy. In the not so distant past, this strategy was only applied in model organisms because there was no feasible technology to thoroughly exploit desired genetic mutations and their impact on drug efficacy in human cells. Today, with the advent of CRISPR gene-editing technology and its application to pooled library screens in mammalian cells, chemogenetic screens are performed directly in human cell lines with high sensitivity and specificity. Chemogenetic profiling provides insights into drug mechanism-of-action, genetic vulnerabilities, and resistance mechanisms, all of which will help to accurately deliver the right drug to the right target in the right patient while minimizing side effects.
Keywords: CRISPR; Chemogenetic screens; Drug-gene interactions.
© 2019 The Authors.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
