Self-assembly of a terbium(III) 1D coordination polymer on mica
- PMID: 31921522
- PMCID: PMC6941415
- DOI: 10.3762/bjnano.10.234
Self-assembly of a terbium(III) 1D coordination polymer on mica
Abstract
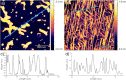
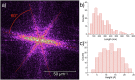
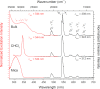
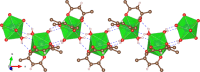
The terbium(III) ion is a particularly suitable candidate for the creation of surface-based magnetic and luminescent devices. In the present work, we report the epitaxial growth of needle-like objects composed of [Tb(hfac)3·2H2O] n (where hfac = hexafluoroacetylacetonate) polymeric units on muscovite mica, which is observed by atomic force microscopy. The needle-like shape mimics the structure observed in the crystalline bulk material. The growth of this molecular organization is assisted by water adsorption on the freshly air-cleaved muscovite mica. This deposition technique allows for the observation of a significant amount of nanochains grown along three preferential directions 60° apart from another. The magnetic properties and the luminescence of the nanochains can be detected without the need of surface-dedicated instrumentation. The intermediate value of the observed luminescence lifetime of the deposits (132 µs) compared to that of the bulk (375 µs) and the CHCl3 solution (13 µs) further reinforces the idea of water-induced growth.
Keywords: atomic force microscopy (AFM); luminescence; nanostructuration; polymer; self-assembly; surface; terbium complexes.
Copyright © 2019, Evrard et al.; licensee Beilstein-Institut.
Figures



References
-
- Lan Y, Klyatskaya S, Ruben M. Bis(phthalocyaninato) Lanthanide(III) Complexes – from Molecular Magnetism to Spintronic Devices. In: Layfield R A, editor. Lanthanides and Actinides in Molecular Magnetism. Weinheim, Germany: Wiley-VCH; 2015. pp. 223–292. - DOI
-
- Hirota E, Sakakima H, Inomata K. Giant Magneto-Resistance Devices. Berlin, Germany: Springer Berlin; 2002. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
