Taiwanin E Induces Cell Cycle Arrest and Apoptosis in Arecoline/4-NQO-Induced Oral Cancer Cells Through Modulation of the ERK Signaling Pathway
- PMID: 31921618
- PMCID: PMC6928190
- DOI: 10.3389/fonc.2019.01309
Taiwanin E Induces Cell Cycle Arrest and Apoptosis in Arecoline/4-NQO-Induced Oral Cancer Cells Through Modulation of the ERK Signaling Pathway
Abstract
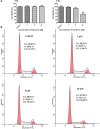
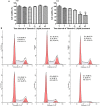
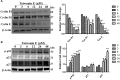
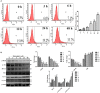
Taiwanin E is a bioactive compound extracted from Taiwania cryptomerioides Hayata. In this research endeavor, we studied the anti-cancer effect of Taiwanin E against arecoline and 4-nitroquinoline-1-oxide-induced oral squamous cancer cells (OSCC), and elucidated the underlying intricacies. OSCC were treated with Taiwanin E and analyzed through MTT assay, Flow cytometry, TUNEL assay, and Western blotting for their efficacy against OSCC. Interestingly, it was found that Taiwanin E significantly attenuated the cell viability of oral cancer cells (T28); however, no significant cytotoxic effects were found for normal oral cells (N28). Further, Flow cytometry analysis showed that Taiwanin E induced G1cell cycle arrest in T28 oral cancer cells and Western blot analysis suggested that Taiwanin E considerably downregulated cell cycle regulatory proteins and activated p53, p21, and p27 proteins. Further, TUNEL and Western blot studies instigated that it induced cellular apoptosis and attenuated the p-PI3K/p-Akt survival mechanism in T28 oral cancer cells seemingly through modulation of the ERK signaling cascade. Collectively, the present study highlights the prospective therapeutic efficacy of Taiwanin E against arecoline and 4-nitroquinoline-1-oxide-induced oral cancer.
Keywords: Taiwanin E; apoptosis; cell cycle arrest; oral cancer; therapeutics.
Copyright © 2019 Wang, Wu, Badrealam, Kuo, Chao, Hsu, Bau, Viswanadha, Chen, Lio, Chiang and Huang.
Figures

Similar articles
-
Down-regulation of β-catenin and the associated migration ability by Taiwanin C in arecoline and 4-NQO-induced oral cancer cells via GSK-3β activation.Mol Carcinog. 2017 Mar;56(3):1055-1067. doi: 10.1002/mc.22570. Epub 2016 Oct 4. Mol Carcinog. 2017. PMID: 27648737
-
Taiwanin C elicits apoptosis in arecoline and 4-nitroquinoline-1-oxide-induced oral squamous cell carcinoma cells and hinders proliferation via epidermal growth factor receptor/PI3K suppression.Environ Toxicol. 2019 Jun;34(6):760-767. doi: 10.1002/tox.22742. Epub 2019 Mar 18. Environ Toxicol. 2019. PMID: 30884126
-
Taiwanin C selectively inhibits arecoline and 4-NQO-induced oral cancer cell proliferation via ERK1/2 inactivation.Environ Toxicol. 2017 Jan;32(1):62-69. doi: 10.1002/tox.22212. Epub 2015 Nov 5. Environ Toxicol. 2017. PMID: 26537528
-
Taiwanin A induced cell cycle arrest and p53-dependent apoptosis in human hepatocellular carcinoma HepG2 cells.Life Sci. 2007 Jan 9;80(5):493-503. doi: 10.1016/j.lfs.2006.10.017. Epub 2006 Dec 19. Life Sci. 2007. PMID: 17182066
-
Taiwanin A inhibits MCF-7 cancer cell activity through induction of oxidative stress, upregulation of DNA damage checkpoint kinases, and activation of p53 and FasL/Fas signaling pathways.Phytomedicine. 2010 Dec 15;18(1):16-24. doi: 10.1016/j.phymed.2010.06.005. Epub 2010 Jul 16. Phytomedicine. 2010. PMID: 20637573
Cited by
-
Prediction of Rab5B inhibitors through integrative in silico techniques.Mol Divers. 2024 Aug;28(4):2547-2562. doi: 10.1007/s11030-023-10693-9. Epub 2023 Jul 28. Mol Divers. 2024. PMID: 37505376
-
Alcalase Potato Protein Hydrolysate-PPH902 Enhances Myogenic Differentiation and Enhances Skeletal Muscle Protein Synthesis under High Glucose Condition in C2C12 Cells.Molecules. 2021 Oct 30;26(21):6577. doi: 10.3390/molecules26216577. Molecules. 2021. PMID: 34770984 Free PMC article.
-
Bioinformatics Analysis and Experimental Validation of Epigallocatechin-3-gallate Against Iopromide-induced Injury in HEK-293 Cells via Anti-oxidative and Anti-inflammation Pathways.In Vivo. 2024 Nov-Dec;38(6):2617-2628. doi: 10.21873/invivo.13738. In Vivo. 2024. PMID: 39477405 Free PMC article.
-
Roles of TRPM channels in glioma.Cancer Biol Ther. 2024 Dec 31;25(1):2338955. doi: 10.1080/15384047.2024.2338955. Epub 2024 Apr 29. Cancer Biol Ther. 2024. PMID: 38680092 Free PMC article. Review.
-
Total synthesis of justicidin B, justicidin E, and taiwanin C: A general and flexible approach toward the synthesis of natural arylnaphthalene lactone lignans.Front Chem. 2022 Dec 22;10:1103554. doi: 10.3389/fchem.2022.1103554. eCollection 2022. Front Chem. 2022. PMID: 36618865 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

