Expression and Function of the Endocannabinoid Modulating Enzymes Fatty Acid Amide Hydrolase and N-Acylphosphatidylethanolamine-Specific Phospholipase D in Endometrial Carcinoma
- PMID: 31921630
- PMCID: PMC6930916
- DOI: 10.3389/fonc.2019.01363
Expression and Function of the Endocannabinoid Modulating Enzymes Fatty Acid Amide Hydrolase and N-Acylphosphatidylethanolamine-Specific Phospholipase D in Endometrial Carcinoma
Abstract
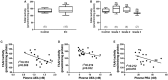
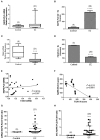
Background: The concentrations of three N-acylethanolamines (NAEs), anandamide (AEA), N-oleoylethanolamide (OEA), and N-palmitylethanolamide (PEA) are increased in the endometria of women with endometrial cancer (EC). It is widely accepted that plasma levels of these three NAEs are regulated by the actions of the rate-limiting enzymes N-acylphoshatidylethanolamine-specific phospholipase D (NAPE-PLD) and fatty acid amide hydrolase (FAAH), which are synthesizing and degradative, respectively. The expression and activity of these enzymes have not previously been studied in EC. Methods: FAAH activity in peripheral blood lymphocytes, and transcript and protein expression for FAAH and NAPE-PLD in EC tissues were measured using enzyme, quantitative RT-PCR, and histomorphometry (of immunoreactive tissue sections), respectively. Samples were from 6 post-menopausal women with atrophic endometria (controls) and 34 women with histologically diagnosed EC. Concentrations of the three NAEs also measured in plasma and tissues were correlated with lymphocytic FAAH activity and the NAPE-PLD and FAAH transcript and protein levels. Results: Peripheral lymphocyte FAAH activity was unaffected in women with EC compared to controls. The FAAH transcript expression level was significantly (p < 0.0001) 75% lower in EC whilst NAPE-PLD levels were not significantly (p = 0.798) increased. In line with the transcript data, a significant (p < 0.0001) tumor type-dependent 70-90% decrease in FAAH protein and significant 4- to 14-fold increase in NAPE-PLD protein (p < 0.0001) was observed in the malignant tissue with more advanced disease having lower FAAH and higher NAPE-PLD expression than less advanced disease. Correlation analyses also confirmed that tissue NAE concentrations were inversely related to FAAH expression and directly correlated to NAPE-PLD expression and the NAPE-PLD/FAAH ratio. Conclusion: These data support our previous observation of tissue levels of AEA, OEA, and PEA and a role for NAE metabolism in the pathogenesis of EC.
Keywords: N-acylphoshatidylethanolmine-specific phospholipase D; endocannabinoids; endometrial cancer; fatty acid amide hydrolase; gene expression; immunohistochemistry.
Copyright © 2019 Ayakannu, Taylor, Bari, Mastrangelo, Maccarrone and Konje.
Figures
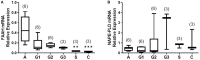



References
-
- Matias I, Di Marzo V. Endocannabinoid synthesis and degradation, and their regulation in the framework of energy balance. J Endocrinol Invest. (2006) 29:15–26. - PubMed
LinkOut - more resources
Full Text Sources

