Technology Advancements in Blood Coagulation Measurements for Point-of-Care Diagnostic Testing
- PMID: 31921804
- PMCID: PMC6917661
- DOI: 10.3389/fbioe.2019.00395
Technology Advancements in Blood Coagulation Measurements for Point-of-Care Diagnostic Testing
Abstract
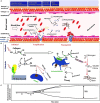
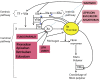
In recent years, blood coagulation monitoring has become crucial to diagnosing causes of hemorrhages, developing anticoagulant drugs, assessing bleeding risk in extensive surgery procedures and dialysis, and investigating the efficacy of hemostatic therapies. In this regard, advanced technologies such as microfluidics, fluorescent microscopy, electrochemical sensing, photoacoustic detection, and micro/nano electromechanical systems (MEMS/NEMS) have been employed to develop highly accurate, robust, and cost-effective point of care (POC) devices. These devices measure electrochemical, optical, and mechanical parameters of clotting blood. Which can be correlated to light transmission/scattering, electrical impedance, and viscoelastic properties. In this regard, this paper discusses the working principles of blood coagulation monitoring, physical and sensing parameters in different technologies. In addition, we discussed the recent progress in developing nanomaterials for blood coagulation detection and treatments which opens up new area of controlling and monitoring of coagulation at the same time in the future. Moreover, commercial products, future trends/challenges in blood coagulation monitoring including novel anticoagulant therapies, multiplexed sensing platforms, and the application of artificial intelligence in diagnosis and monitoring have been included.
Keywords: MEMS; POC devices; blood coagulation; electrochemical sensing; fluorescent microscopy; microfluidics; nanomaterials; photoacoustic detection.
Copyright © 2019 Mohammadi Aria, Erten and Yalcin.
Figures



References
-
- Andersson M., Andersson J., Sellborn A., Berglin M., Nilsson B., Elwing H. (2005). Quartz crystal microbalance-with dissipation monitoring (Qcm-D) for real time measurements of blood coagulation density and immune complement activation on artificial surfaces. Biosens. Bioelectron. 21, 79–86. 10.1016/j.bios.2004.09.026 - DOI - PubMed
-
- Aria M. M., Irajizad A., Astaraei F. R., Shariatpanahi S. P., Sarvari R. (2016). Ethanol sensing properties of Pvp electrospun membranes studied by quartz crystal microbalance. Measurement 78, 283–288. 10.1016/j.measurement.2015.10.018 - DOI
-
- Baryshnikova E., Di Dedda U., Ranucci M. (2019). A comparative study of seer sonorheometry versus standard coagulation tests, rotational thromboelastometry, and multiple electrode aggregometry in cardiac surgery. J. Cardiothorac. Vasc. Anesth. 33, 1590–1598. 10.1053/j.jvca.2019.01.011 - DOI - PubMed
-
- Berney H., O'Riordan J. J. (2008). Impedance measurement monitors blood coagulation. Anal. Dial. 42, 42–08.
-
- Bernhardt I., Wesseling M. C., Nguyen D. B., Kaestner L. (2019). Red blood cells actively contribute to blood coagulation and thrombus formation. Erythrocyte. Intechopen. 10.5772/intechopen.86152 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

