Analysis of the Effectiveness of Sub-sensory Electrical Noise Stimulation During Visuomotor Adaptations in Different Visual Feedback Conditions
- PMID: 31921805
- PMCID: PMC6915077
- DOI: 10.3389/fbioe.2019.00399
Analysis of the Effectiveness of Sub-sensory Electrical Noise Stimulation During Visuomotor Adaptations in Different Visual Feedback Conditions
Abstract
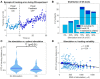
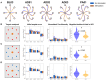
Sub-sensory electrical noise stimulation has been shown to improve motor performance in tasks that mainly rely on proprioceptive feedback. During the execution of movements such as reaching, proprioceptive feedback combines dynamically with visual feedback. It is still unclear whether boosting proprioceptive information in tasks where proprioception mixes with vision can influence motor performance. To better understand this point, we tested the effect of electrical noise stimulation applied superficially to the muscle spindles during four different experiments consisting of isometric reaching tasks under different visual feedback conditions. The first experiment (n = 40) consisted of a reach-and-hold task where subjects had to hold a cursor on a target for 30 s and had visual feedback removed 10 s into the task. Subjects performed 30 repetitions of this task with different stimulation levels, including no stimulation. We observed that trials in which the stimulation was present displayed smaller movement variability. Moreover, we observed a positive correlation between the level of stimulation and task performance. The other three experiments consisted of three versions of an isometric visuomotor adaptation task where subjects were asked to reach to random targets in <1.5 s (otherwise incurring in negative feedback) while overcoming a 45° clockwise rotation in the mapping between the force exerted and the movement of the cursor. The three experiments differed in the visual feedback presented to the subjects, with one group (n = 20) performing the experiment with full visual feedback, one (n = 10) with visual feedback restricted only to the beginning of the trajectory, and one (n = 10) without visual feedback of the trajectory. All subjects performed their experiment twice, with and without stimulation. We did not observe substantial effects of the stimulation when visual feedback was present (either completely or partially). We observed a limited effect of the stimulation in the absence of visual feedback consisting in a significant smaller number of negative-feedback trials and a significant smaller movement time in the first block of the adaptation phase. Our results suggest that sub-sensory stimulation can be beneficial when proprioception is the main feedback modality but mostly ineffective in tasks where visual feedback is actively employed.
Keywords: motor control; proprioception; stochastic resonance; visual feedback; visuomotor adaptation.
Copyright © 2019 Castronovo, Giles Doran, Holden and Severini.
Figures





References
LinkOut - more resources
Full Text Sources

