Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development
- PMID: 31921823
- PMCID: PMC6932962
- DOI: 10.3389/fbioe.2019.00420
Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development
Abstract
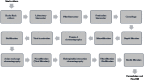
Infectious diseases, along with cancers, are among the main causes of death among humans worldwide. The production of therapeutic proteins for treating diseases at large scale for millions of individuals is one of the essential needs of mankind. Recent progress in the area of recombinant DNA technologies has paved the way to producing recombinant proteins that can be used as therapeutics, vaccines, and diagnostic reagents. Recombinant proteins for these applications are mainly produced using prokaryotic and eukaryotic expression host systems such as mammalian cells, bacteria, yeast, insect cells, and transgenic plants at laboratory scale as well as in large-scale settings. The development of efficient bioprocessing strategies is crucial for industrial production of recombinant proteins of therapeutic and prophylactic importance. Recently, advances have been made in the various areas of bioprocessing and are being utilized to develop effective processes for producing recombinant proteins. These include the use of high-throughput devices for effective bioprocess optimization and of disposable systems, continuous upstream processing, continuous chromatography, integrated continuous bioprocessing, Quality by Design, and process analytical technologies to achieve quality product with higher yield. This review summarizes recent developments in the bioprocessing of recombinant proteins, including in various expression systems, bioprocess development, and the upstream and downstream processing of recombinant proteins.
Keywords: continuous chromatography; downstream processing; high-throughput technology; integrated continuous bioprocessing; perfusion cell culture; process development; protein expression; upstream processing.
Copyright © 2019 Tripathi and Shrivastava.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

