Signaling Enzymes Required for Sperm Maturation and Fertilization in Mammals
- PMID: 31921853
- PMCID: PMC6930163
- DOI: 10.3389/fcell.2019.00341
Signaling Enzymes Required for Sperm Maturation and Fertilization in Mammals
Abstract
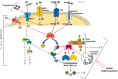
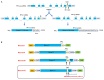
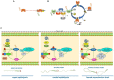
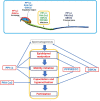
In mammals, motility and fertilizing ability of spermatozoa develop during their passage through the epididymis. After ejaculation, sperm undergo capacitation and hyperactivation in the female reproductive tract - a motility transition that is required for sperm penetration of the egg. Both epididymal initiation of sperm motility and hyperactivation are essential for male fertility. Motility initiation in the epididymis and sperm hyperactivation involve changes in metabolism, cAMP (cyclic adenosine mono-phosphate), calcium and pH acting through protein kinases and phosphatases. Despite this knowledge, we still do not understand, in biochemical terms, how sperm acquire motility in the epididymis and how motility is altered in the female reproductive tract. Recent data show that the sperm specific protein phosphatase PP1γ2, glycogen synthase kinase 3 (GSK3), and the calcium regulated phosphatase calcineurin (PP2B), are involved in epididymal sperm maturation. The protein phosphatase PP1γ2 is present only in testis and sperm in mammals. PP1γ2 has a isoform-specific requirement for normal function of mammalian sperm. Sperm PP1γ2 is regulated by three proteins - inhibitor 2, inhibitor 3 and SDS22. Changes in phosphorylation of these three inhibitors and their binding to PP1γ2 are involved in initiation and activation of sperm motility. The inhibitors are phosphorylated by protein kinases, one of which is GSK3. The isoform GSK3α is essential for epididymal sperm maturation and fertility. Calcium levels dramatically decrease during sperm maturation and initiation of motility suggesting that the calcium activated sperm phosphatase (PP2B) activity also decreases. Loss of PP2B results in male infertility due to impaired sperm maturation in the epididymis. Thus the three signaling enzymes PP1γ2, GSK3, and PP2B along with the documented PKA (protein kinase A) have key roles in sperm maturation and hyperactivation. Significantly, all these four signaling enzymes are present as specific isoforms only in placental mammals, a testimony to their essential roles in the unique aspects of sperm function in mammals. These findings should lead to a better biochemical understanding of the basis of male infertility and should lead to novel approaches to a male contraception and managed reproduction.
Keywords: GSK3α; PKA; PP1γ2; PP2B; contraception; epididymal sperm maturation; fertility; hyperactivation.
Copyright © 2019 Dey, Brothag and Vijayaraghavan.
Figures

References
-
- Allouche-Fitoussi D., Bakhshi D., Breitbart H. (2018). Signaling pathways involved in human sperm hyperactivated motility stimulated by Zn(2). Mol. Reprod. Dev. 85 543–556. - PubMed
-
- Alon L. T., Pietrokovski S., Barkan S., Avrahami L., Kaidanovich-Beilin O., Woodgett J. R., et al. (2011). Selective loss of glycogen synthase kinase-3alpha in birds reveals distinct roles for GSK-3 isozymes in tau phosphorylation. FEBS Lett. 585 1158–1162. 10.1016/j.febslet.2011.03.025 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

