Active Sleep Promotes Coherent Oscillatory Activity in the Cortico-Hippocampal System of Infant Rats
- PMID: 31922194
- PMCID: PMC7175014
- DOI: 10.1093/cercor/bhz223
Active Sleep Promotes Coherent Oscillatory Activity in the Cortico-Hippocampal System of Infant Rats
Abstract
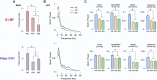
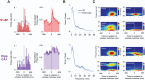
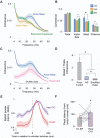
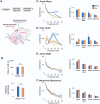
Active sleep (AS) provides a unique developmental context for synchronizing neural activity within and between cortical and subcortical structures. In week-old rats, sensory feedback from myoclonic twitches, the phasic motor activity that characterizes AS, promotes coherent theta oscillations (4-8 Hz) in the hippocampus and red nucleus, a midbrain motor structure. Sensory feedback from twitches also triggers rhythmic activity in sensorimotor cortex in the form of spindle bursts, which are brief oscillatory events composed of rhythmic components in the theta, alpha/beta (8-20 Hz), and beta2 (20-30 Hz) bands. Here we ask whether one or more of these spindle-burst components are communicated from sensorimotor cortex to hippocampus. By recording simultaneously from whisker barrel cortex and dorsal hippocampus in 8-day-old rats, we show that AS, but not other behavioral states, promotes cortico-hippocampal coherence specifically in the beta2 band. By cutting the infraorbital nerve to prevent the conveyance of sensory feedback from whisker twitches, cortical-hippocampal beta2 coherence during AS was substantially reduced. These results demonstrate the necessity of sensory input, particularly during AS, for coordinating rhythmic activity between these two developing forebrain structures.
Keywords: REM sleep; barrel cortex; development; functional connectivity; myoclonic twitching.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

