Neural stem cell transplantation improves learning and memory by protecting cholinergic neurons and restoring synaptic impairment in an amyloid precursor protein/presenilin 1 transgenic mouse model of Alzheimer's disease
- PMID: 31922229
- PMCID: PMC7002968
- DOI: 10.3892/mmr.2020.10918
Neural stem cell transplantation improves learning and memory by protecting cholinergic neurons and restoring synaptic impairment in an amyloid precursor protein/presenilin 1 transgenic mouse model of Alzheimer's disease
Abstract
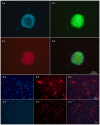
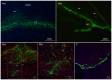
Alzheimer's disease (AD) is the most prevalent age‑related neurodegenerative disorder. It is featured by the progressive accumulation of β‑amyloid (Aβ) plaques and neurofibrillary tangles. This can eventually lead to a decrease of cholinergic neurons in the basal forebrain. Stem cell transplantation is an effective treatment for neurodegenerative diseases. Previous studies have revealed that different types of stem or progenitor cells can mitigate cognition impairment in different Alzheimer's disease mouse models. However, understanding the underlying mechanisms of neural stem cell (NSC) therapies for AD requires further investigation. In the present study, the effects and the underlying mechanisms of the treatment of AD by NSCs are reported. The latter were labelled with the enhanced green fluorescent protein (EGFP) prior to implantation into the bilateral hippocampus of an amyloid precursor protein (APP)/presenilin 1 (PS1) transgenic (Tg) mouse model of AD. It was observed that the number of basal forebrain cholinergic neurons was restored and the expression of choline acetyltransferase (ChAT) protein was increased. Moreover, the levels of synaptophysin (SYP), postsynaptic density protein 95 (PSD‑95) and microtubule‑associated protein (MAP‑2) were significantly increased in the hippocampus of NSC‑treated AD mice. Notably, spatial learning and memory were both improved after transplantation of NSCs. In conclusion, the present study revealed that NSC transplantation improved learning and memory functions in an AD mouse model. This treatment allowed repairing of basal forebrain cholinergic neurons and increased the expression of the cognition‑related proteins SYP, PSD‑95 and MAP‑2 in the hippocampus.
Keywords: alzheimer's disease; neural stem cell; cholinergic neurons; synapse; transplantation.
Figures



References
-
- Teng YD. Functional multipotency of stem cells: Biological traits gleaned from neural progeny studies. Semin Cell Dev Biol. 2019;pii:S1084–S9521. (Epub ahead of print) - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

