Two forms of Opa1 cooperate to complete fusion of the mitochondrial inner-membrane
- PMID: 31922487
- PMCID: PMC7299343
- DOI: 10.7554/eLife.50973
Two forms of Opa1 cooperate to complete fusion of the mitochondrial inner-membrane
Abstract
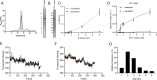
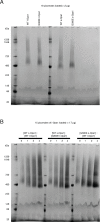
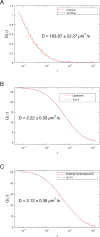
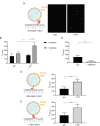
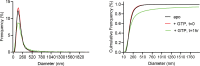
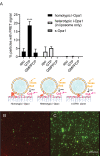
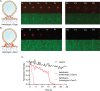
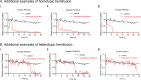
Mitochondrial membrane dynamics is a cellular rheostat that relates metabolic function and organelle morphology. Using an in vitro reconstitution system, we describe a mechanism for how mitochondrial inner-membrane fusion is regulated by the ratio of two forms of Opa1. We found that the long-form of Opa1 (l-Opa1) is sufficient for membrane docking, hemifusion and low levels of content release. However, stoichiometric levels of the processed, short form of Opa1 (s-Opa1) work together with l-Opa1 to mediate efficient and fast membrane pore opening. Additionally, we found that excess levels of s-Opa1 inhibit fusion activity, as seen under conditions of altered proteostasis. These observations describe a mechanism for gating membrane fusion.
Keywords: fusion; in vitro reconstitution; membranes; mitochondria; molecular biophysics; none; structural biology.
© 2020, Ge et al.
Conflict of interest statement
YG, XS, SB, JM, AS, LC No competing interests declared
Figures









References
-
- Abutbul-Ionita I, Rujiviphat J, Nir I, McQuibban GA, Danino D. Membrane tethering and Nucleotide-dependent conformational changes drive mitochondrial genome maintenance (Mgm1) Protein-mediated membrane fusion. Journal of Biological Chemistry. 2012;287:36634–36638. doi: 10.1074/jbc.C112.406769. - DOI - PMC - PubMed
-
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nature Genetics. 2000;26:211–215. doi: 10.1038/79944. - DOI - PubMed
-
- Antonny B, Burd C, De Camilli P, Chen E, Daumke O, Faelber K, Ford M, Frolov VA, Frost A, Hinshaw JE, Kirchhausen T, Kozlov MM, Lenz M, Low HH, McMahon H, Merrifield C, Pollard TD, Robinson PJ, Roux A, Schmid S. Membrane fission by dynamin: what we know and what we need to know. The EMBO Journal. 2016;35:2270–2284. doi: 10.15252/embj.201694613. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

