Ultrasound-Triggered Enzymatic Gelation
- PMID: 31922627
- PMCID: PMC7180077
- DOI: 10.1002/adma.201905914
Ultrasound-Triggered Enzymatic Gelation
Abstract
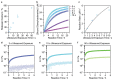
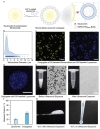
Hydrogels are formed using various triggers, including light irradiation, pH adjustment, heating, cooling, or chemical addition. Here, a new method for forming hydrogels is introduced: ultrasound-triggered enzymatic gelation. Specifically, ultrasound is used as a stimulus to liberate liposomal calcium ions, which then trigger the enzymatic activity of transglutaminase. The activated enzyme catalyzes the formation of fibrinogen hydrogels through covalent intermolecular crosslinking. The catalysis and gelation processes are monitored in real time and both the enzyme kinetics and final hydrogel properties are controlled by varying the initial ultrasound exposure time. This technology is extended to microbubble-liposome conjugates, which exhibit a stronger response to the applied acoustic field and are also used for ultrasound-triggered enzymatic hydrogelation. To the best of the knowledge, these results are the first instance in which ultrasound is used as a trigger for either enzyme catalysis or enzymatic hydrogelation. This approach is highly versatile and can be readily applied to different ion-dependent enzymes or gelation systems. Moreover, this work paves the way for the use of ultrasound as a remote trigger for in vivo hydrogelation.
Keywords: enzymes; hydrogels; liposomes; microbubbles; ultrasound.
© 2020 The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
V.N., J.P.K.A., C.E.S., and M.M.S. are inventors on a patent application related to the technology described in this paper.
Figures

References
MeSH terms
Substances
Grants and funding
- 21138/Arthritis Research U.K. Foundation
- RE/13/4/30184/BHF_/British Heart Foundation/United Kingdom
- DMR-0520547/NSF
- MR/R015651/1/UK Regenerative Medicine Platform
- Whitaker International Program
- Institute of International Education
- EP/K020641/1/Engineering and Physical Sciences Research Council
- MR/K026682/1/MRC_/Medical Research Council/United Kingdom
- MR/S00551X/1/MRC_/Medical Research Council/United Kingdom
- MR/K026666/1/MRC_/Medical Research Council/United Kingdom
- 654000/European Union's Horizon 2020 research and innovation programme under the SINE2020
- RB1810203/Science and Technology Facilities Council
- Rosetrees Trust
- 21138/VAC_/Versus Arthritis/United Kingdom
- Ermenegildo Zegna Founder's Scholarship program
- MR/K026682/1/UK Regenerative Medicine Platform
- MR/R015651/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

